Spatiotemporal coordination of Greatwall-Endos-PP2A promotes mitotic progression
- PMID: 33836042
- PMCID: PMC8042607
- DOI: 10.1083/jcb.202008145
Spatiotemporal coordination of Greatwall-Endos-PP2A promotes mitotic progression
Abstract
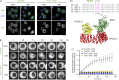
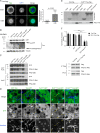
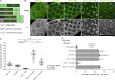
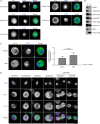
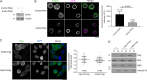
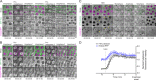
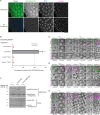
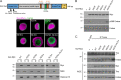
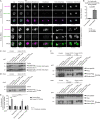
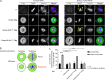
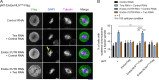
Mitotic entry involves inhibition of protein phosphatase 2A bound to its B55/Tws regulatory subunit (PP2A-B55/Tws), which dephosphorylates substrates of mitotic kinases. This inhibition is induced when Greatwall phosphorylates Endos, turning it into an inhibitor of PP2A-Tws. How this mechanism operates spatiotemporally in the cell is incompletely understood. We previously reported that the nuclear export of Greatwall in prophase promotes mitotic progression. Here, we examine the importance of the localized activities of PP2A-Tws and Endos for mitotic regulation. We find that Tws shuttles through the nucleus via a conserved nuclear localization signal (NLS), but expression of Tws in the cytoplasm and not in the nucleus rescues the development of tws mutants. Moreover, we show that Endos must be in the cytoplasm before nuclear envelope breakdown (NEBD) to be efficiently phosphorylated by Greatwall and to bind and inhibit PP2A-Tws. Disrupting the cytoplasmic function of Endos before NEBD results in subsequent mitotic defects. Evidence suggests that this spatiotemporal regulation is conserved in humans.
© 2021 Larouche et al.
Figures



References
-
- Álvarez-Fernández, M., Sánchez-Martínez R., Sanz-Castillo B., Gan P.P., Sanz-Flores M., Trakala M., Ruiz-Torres M., Lorca T., Castro A., and Malumbres M.. 2013. Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc. Natl. Acad. Sci. USA. 110:17374–17379. 10.1073/pnas.1310745110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

