Neuromechanical wave resonance in jellyfish swimming
- PMID: 33836589
- PMCID: PMC7980403
- DOI: 10.1073/pnas.2020025118
Neuromechanical wave resonance in jellyfish swimming
Abstract
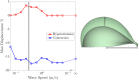
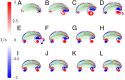
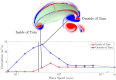
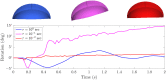
For organisms to have robust locomotion, their neuromuscular organization must adapt to constantly changing environments. In jellyfish, swimming robustness emerges when marginal pacemakers fire action potentials throughout the bell's motor nerve net, which signals the musculature to contract. The speed of the muscle activation wave is dictated by the passage times of the action potentials. However, passive elastic material properties also influence the emergent kinematics, with time scales independent of neuromuscular organization. In this multimodal study, we examine the interplay between these two time scales during turning. A three-dimensional computational fluid-structure interaction model of a jellyfish was developed to determine the resulting emergent kinematics, using bidirectional muscular activation waves to actuate the bell rim. Activation wave speeds near the material wave speed yielded successful turns, with a 76-fold difference in turning rate between the best and worst performers. Hyperextension of the margin occurred only at activation wave speeds near the material wave speed, suggesting resonance. This hyperextension resulted in a 34-fold asymmetry in the circulation of the vortex ring between the inside and outside of the turn. Experimental recording of the activation speed confirmed that jellyfish actuate within this range, and flow visualization using particle image velocimetry validated the corresponding fluid dynamics of the numerical model. This suggests that neuromechanical wave resonance plays an important role in the robustness of an organism's locomotory system and presents an undiscovered constraint on the evolution of flexible organisms. Understanding these dynamics is essential for developing actuators in soft body robotics and bioengineered pumps.
Keywords: fluid–structure interaction; jellyfish; maneuverability; neuromechanics; propulsion.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Stewart W. J., Cardenas G. S., McHenry M. J., Zebrafish larvae evade predators by sensing water flow. J. Exp. Biol. 216, 388–398 (2013). - PubMed
-
- Mackie G. O., Central neural circuitry in the jellyfish Aglantha: A model ‘simple nervous system’. Neurosignals 13, 5–19 (2004). - PubMed
-
- Olberg R., Worthington A., Venator K., Prey pursuit and interception in dragonflies. J. Comp. Physiol. 186, 155–162 (2000). - PubMed
-
- Drucker E., Lauder G., Wake dynamics and fluid forces of turning maneuvers in sunfish. J. Exp. Biol. 204, 431–442 (2001). - PubMed
-
- Muijres F. T., Elzinga M. J., Melis J. M., Dickinson M. H., Flies evade looming targets by executing rapid visually directed banked turns. Science 344, 172–177 (2014). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

