Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension
- PMID: 33836606
- PMCID: PMC7980441
- DOI: 10.1073/pnas.2023899118
Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension
Abstract
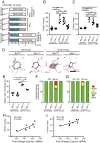
Pulmonary arterial hypertension (PAH) is a devastating disease characterized by arteriopathy in the small to medium-sized distal pulmonary arteries, often accompanied by infiltration of inflammatory cells. Aryl hydrocarbon receptor (AHR), a nuclear receptor/transcription factor, detoxifies xenobiotics and regulates the differentiation and function of various immune cells. However, the role of AHR in the pathogenesis of PAH is largely unknown. Here, we explore the role of AHR in the pathogenesis of PAH. AHR agonistic activity in serum was significantly higher in PAH patients than in healthy volunteers and was associated with poor prognosis of PAH. Sprague-Dawley rats treated with the potent endogenous AHR agonist, 6-formylindolo[3,2-b]carbazole, in combination with hypoxia develop severe pulmonary hypertension (PH) with plexiform-like lesions, whereas Sprague-Dawley rats treated with the potent vascular endothelial growth factor receptor 2 inhibitors did not. Ahr-knockout (Ahr-/- ) rats generated using the CRISPR/Cas9 system did not develop PH in the SU5416/hypoxia model. A diet containing Qing-Dai, a Chinese herbal drug, in combination with hypoxia led to development of PH in Ahr+/+ rats, but not in Ahr-/- rats. RNA-seq analysis, chromatin immunoprecipitation (ChIP)-seq analysis, immunohistochemical analysis, and bone marrow transplantation experiments show that activation of several inflammatory signaling pathways was up-regulated in endothelial cells and peripheral blood mononuclear cells, which led to infiltration of CD4+ IL-21+ T cells and MRC1+ macrophages into vascular lesions in an AHR-dependent manner. Taken together, AHR plays crucial roles in the development and progression of PAH, and the AHR-signaling pathway represents a promising therapeutic target for PAH.
Keywords: Qing-Dai; aryl hydrocarbon receptor (AHR); inflammation; pulmonary arterial hypertension; transcription factor.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: R.A. and T.O. belong to a department endowed by funding from Nippon Shinyaku Co., Ltd.
Figures








References
-
- Humbert M., et al. ., Cellular and molecular pathobiology of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 43(12, suppl. S)13S–24S (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

