Trends in oral corticosteroids use in severe asthma: a 14-year population-based study
- PMID: 33836765
- PMCID: PMC8034163
- DOI: 10.1186/s12931-021-01696-x
Trends in oral corticosteroids use in severe asthma: a 14-year population-based study
Abstract
Background: Oral corticosteroids are important components of pharmacotherapy in severe asthma. Our objective was to describe the extent, trends, and factors associated with exposure to oral corticosteroids (OCS) in a severe asthma cohort.
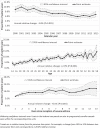
Methods: We used administrative health databases of British Columbia, Canada (2000-2014) and validated algorithms to retrospectively create a cohort of severe asthma patients. Exposure to OCS within each year of follow-up was measured in two ways: maintenance use as receiving on average ≥ 2.5 mg/day (prednisone-equivalent) OCS, and episodic use as the number of distinct episodes of OCS exposure for up to 14 days. Trends and factors associated with exposure on three time axes (calendar year, age, and time since diagnosis) were evaluated using Poisson regression.
Results: 21,144 patients (55.4% female; mean entry age 28.7) contributed 40,803 follow-up years, in 8.2% of which OCS was used as maintenance therapy. Maintenance OCS use declined by 3.8%/calendar year (p < 0.001). The average number of episodes of OCS use was 0.89/year, which increased by 1.1%/calendar year (p < 0.001). Trends remained significant for both exposure types in adjusted analyses. Both maintenance and episodic use increased by age and time since diagnosis.
Conclusions: This population-based study documented a secular downward trend in maintenance OCS use in a period before widespread use of biologics. This might have been responsible for a higher rate of exacerbations that required episodic OCS therapy. Such trends in OCS use might be due to changes in the epidemiology of severe asthma, or changes in patient and provider preferences over time.
Keywords: Asthma; Asthma/*drug therapy; Cohort Studies; Corticosteroids; Drug Prescriptions/*statistics & numerical data.
Conflict of interest statement
JMF is a member of the Global Initiative for Asthma (GINA) Executive and Science Committees. He has served on advisory boards for Novartis, Pfizer, AstraZeneca, Boehringer-Ingelheim, and Merck. He has also been a member of speakers’ bureaus for AstraZeneca, Boehringer-Ingelheim, Novartis, and Merck. He has received research funding paid directly to the University of British Columbia from AstraZeneca, Glaxo-SmithKline, Boehringer-Ingelheim, Merck, Sanofi, and Novartis. MS receives salary support from the Canadian Institutes of Health Research and Michael Smith Foundation for Health Research. He has received speaker fees and honoraria from GlaxoSmithKline, AstraZeneca, Teva, and Boehringer Ingelheim. Other authors have no conflict of interests to declare. He has received research funding paid directly to the University of British Columbia from AstraZeneca and Boehringer-Ingelheim.
Figures

References
-
- Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4(120–129):e3. - PubMed
-
- Mincheva R, Ekerljung L, Bossios A, Lundbäck B, Lötvall J. High prevalence of severe asthma in a large random population study. J Allergy Clin Immunol. 2018;141(2256):2264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

