A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease
- PMID: 33836798
- PMCID: PMC8035770
- DOI: 10.1186/s13195-021-00809-4
A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer disease
Abstract
Background: Aducanumab is an anti-amyloid-β (Aβ) antibody that achieved reduced amyloid pathology in Alzheimer's disease (AD) trials; however, it is controversial whether it also improved cognition, which has been suggested would require a sufficiently high cumulative dose of the antibody in the brain. Therapeutic ultrasound, in contrast, has only begun to be investigated in human AD clinical trials. We have previously shown that scanning ultrasound in combination with intravenously injected microbubbles (SUS), which temporarily and safely opens the blood-brain barrier (BBB), removes amyloid and restores cognition in APP23 mice. However, there has been no direct testing of how the effects of SUS compare to immunotherapy or whether a combination therapy is more effective.
Methods: In a study comprising four treatment arms, we tested the efficacy of an Aducanumab analog, Adu, both in comparison to SUS, and as a combination therapy, in APP23 mice (aged 13-22 months), using sham as a control. The active place avoidance (APA) test was used to test spatial memory, and histology and ELISA were used to measure amyloid. Brain antibody levels were also determined.
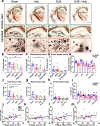
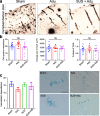
Results: We found that both Adu and SUS reduced the total plaque area in the hippocampus with no additive effect observed with the combination treatment (SUS + Adu). Whereas in the cortex where there was a trend towards reducing the total plaque area from either Adu or SUS, only the combination treatment yielded a statistically significant decrease in total plaque area compared to sham. Only the SUS and SUS + Adu groups included animals that had their plaque load reduced to below 1% from above 10%. There was a robust improvement in spatial memory for the SUS + Adu group only, and in this group the level of Adu, when measured 3 days post-treatment, was 5-fold higher compared to those mice that received Adu on its own. Together, these findings suggest that SUS should be considered as a treatment option for AD. Alternatively, a combination trial using Aducanumab together with ultrasound to increase brain levels of the antibody may be warranted.
Keywords: Alzheimer’s disease; Amyloid-β; Blood–brain barrier; Dementia; Focused ultrasound; Immunotherapy.
Conflict of interest statement
No competing interests exist.
Figures


References
-
- Arndt JW, Qian F, Smith BA, Quan C, Kilambi KP, Bush MW, Walz T, Pepinsky RB, Bussière T, Hamann S, Cameron TO, Weinreb PH. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-beta. Sci Rep. 2018;8(1):6412. doi: 10.1038/s41598-018-24501-0. - DOI - PMC - PubMed
-
- Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

