Bivalirudin as a Systemic Anticoagulant and Flush Solution Additive for Sequential Mitral and Tricuspid Valve Percutaneous Edge-to-Edge Repair in a Patient With Heparin-Induced Thrombocytopenia
- PMID: 33836962
- PMCID: PMC8419205
- DOI: 10.1053/j.jvca.2021.03.004
Bivalirudin as a Systemic Anticoagulant and Flush Solution Additive for Sequential Mitral and Tricuspid Valve Percutaneous Edge-to-Edge Repair in a Patient With Heparin-Induced Thrombocytopenia
Abstract
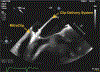
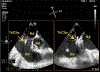
Herein the case of a patient with a prior history of heparin-induced thrombocytopenia who underwent percutaneous mitral valve edge-to-edge repair that was followed by a tricuspid edge-to-edge repair two months later is presented. Recommendations exist for systemic anticoagulant alternatives for percutaneous mitral valve edge-to-edge repair with the MitraClip device (Abbott, Chicago, IL), but minimal guidance and experience are present regarding alternative systemic anticoagulation during the performance of right-sided interventions, including tricuspid edge-to-edge repair (TriClip; Abbott). Notably, there is no clear consensus regarding the use of an alternative anticoagulant in the catheter flush solution for the delivery systems used during these procedures, particularly for right-sided interventions.
Keywords: MitraClip; TriClip; anticoagulation; bivalirudin; heparin-induced thrombocytopenia; percutaneous valve repair.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest The authors declare no competing interests or financial disclosures pertinent to the content of this manuscript. Dr. Ahmed serves as a consultant and proctor for Abbott, Chicago, IL; Edwards Lifesciences, Irvine, CA; and Medtronic, Dublin, Ireland.
Figures

References
-
- U.S. Food and Drug Administration. Premarket approval Mitraclip delivery system. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100009. US Food and Drug Administration Medical Device Databases. Premarket approval number P100009. Approved October 24, 2013.
-
- Rao SV, Ohman EM: Anticoagulant therapy for percutaneous coronary intervention. Circ Cardiovasc Interv. 2010; 3:80–88. - PubMed
-
- U.S. Food and Drug Administration. Prescribing information for heparin sodium for intravenous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/017029s140lbl.pdf. Reference number 4144240. Revised August, 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

