IFNγ Is Critical for CAR T Cell-Mediated Myeloid Activation and Induction of Endogenous Immunity
- PMID: 33837065
- PMCID: PMC8561746
- DOI: 10.1158/2159-8290.CD-20-1661
IFNγ Is Critical for CAR T Cell-Mediated Myeloid Activation and Induction of Endogenous Immunity
Abstract
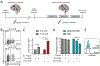
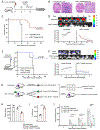
Chimeric antigen receptor (CAR) T cells mediate potent antigen-specific antitumor activity; however, their indirect effects on the endogenous immune system are not well characterized. Remarkably, we demonstrate that CAR T-cell treatment of mouse syngeneic glioblastoma (GBM) activates intratumoral myeloid cells and induces endogenous T-cell memory responses coupled with feed-forward propagation of CAR T-cell responses. IFNγ production by CAR T cells and IFNγ responsiveness of host immune cells are critical for tumor immune landscape remodeling to promote a more activated and less suppressive tumor microenvironment. The clinical relevance of these observations is supported by studies showing that human IL13Rα2-CAR T cells activate patient-derived endogenous T cells and monocytes/macrophages through IFNγ signaling and induce the generation of tumor-specific T-cell responses in a responding patient with GBM. These studies establish that CAR T-cell therapy has the potential to shape the tumor microenvironment, creating a context permissible for eliciting endogenous antitumor immunity. SIGNIFICANCE: Our findings highlight the critical role of IFNγ signaling for a productive CAR T-cell therapy in GBM. We establish that CAR T cells can activate resident myeloid populations and promote endogenous T-cell immunity, emphasizing the importance of host innate and adaptive immunity for CAR T-cell therapy of solid tumors.This article is highlighted in the In This Issue feature, p. 2113.
©2021 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest
Patents associated with IL13Rα2-CAR-T have been licensed by Mustang Bio., Inc., for which S.J.F. and C.E.B. receive royalty payments. All other authors declare no potential conflicts of interest.
Figures





References
-
- Alexander BM, Cloughesy TF. Adult Glioblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35:2402–9 - PubMed
-
- Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. Journal of neuro-oncology 2021;151:41–53 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

