Transarterial radioembolization versus systemic treatment for hepatocellular carcinoma with macrovascular invasion: Analysis of the US National Cancer Database
- PMID: 33837067
- PMCID: PMC8612185
- DOI: 10.2967/jnumed.121.261954
Transarterial radioembolization versus systemic treatment for hepatocellular carcinoma with macrovascular invasion: Analysis of the US National Cancer Database
Abstract
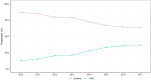
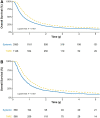
Background and Aims: Systemic therapy remains the recommended first-line treatment for hepatocellular carcinoma (HCC) with macrovascular invasion (MVI). Transarterial radioembolization (TARE) is a promising alternative treatment given superior quality of life. The aims of this study were to 1) characterize trends and correlates for TARE as first-line treatment of HCC patients with MVI in the US and 2) compare survival after TARE versus systemic therapy. Methods: We used the US National Cancer Database to identify patients with T3BN0M0 HCC during 2010-2017. We performed multivariable logistic regression to identify factors associated with use of TARE vs. systemic therapy and Cox proportional hazards regression to identify factors associated with overall survival. Results: Of 11,259 patients with T3BN0M0 HCC, 1454 (12.9%) and 3915 (34.7%) patients were treated with TARE and systemic therapy, respectively. The proportion of patients who received TARE increased from 13.0% in 2010 to 37.0% in 2017. Older age, White race, and receiving care at an academic cancer program were associated with receipt of TARE, while lack of insurance, higher MELD score, Charlson comorbidity Index ≥3, and Northeast region were associated with receipt of systemic therapy. TARE was associated with reduced mortality compared to systemic therapy (adjusted hazard ratio: 0.74, 95%CI: 0.68-0.80), with consistent results observed in propensity weighted analysis and across all examined subgroups. Conclusion: Use of TARE as first-line therapy for HCC with MVI has increased in the US. Patient characteristics, region, and medical center type affected the use of TARE. TARE was associated with reduced mortality compared to systemic therapy for HCC patients with MVI.
Keywords: Hepatology; Oncology: Liver; Radionuclide Therapy; hepatocellular carcinoma; macrovascular invasion; systemic treatment; transarterial radioembolization.
Copyright © 2021 by the Society of Nuclear Medicine and Molecular Imaging, Inc.
Figures

References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
