Effects of an optimised approach to home-based respiratory care in individuals with amyotrophic lateral sclerosis: a study protocol for a randomised controlled trial
- PMID: 33837098
- PMCID: PMC8043034
- DOI: 10.1136/bmjopen-2020-042780
Effects of an optimised approach to home-based respiratory care in individuals with amyotrophic lateral sclerosis: a study protocol for a randomised controlled trial
Abstract
Introduction: This study aims to investigate the effects of an optimal home-based respiratory care protocol in individuals with amyotrophic lateral sclerosis (ALS).
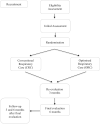
Methods and analysis: This is a randomised, blinded controlled trial involving patients diagnosed with ALS, both sexes, age between 18 and 80 years. Patients will be randomly allocated into the conventional respiratory care (CRC) group and the optimised respiratory care home-based (ORC) group. Primary outcomes will be peak cough flow, the number of exacerbations and ALS Functional Rating Scale Revised. Secondary outcomes will include chest wall volumes, maximal respiratory pressures, sniff nasal inspiratory pressure, nasal expiratory pressure and forced vital capacity (FVC), forced expiratory volume in the 1st second (FEV1) and FEV1/FVC. The CRC group will receive educational information about respiratory care at the clinic. The ORC group will receive conventional care and home-based care. The clinical status of all individuals will be monitored weekly through telephone calls. A 6-month intervention is planned, the outcomes will be assessed every 3 months and 3 and 6 months follow-up after final evaluation. The primary and secondary results will be described as average or median for continuous variables and absolute and relative frequencies for qualitative variables. Treatment effects or differences between the outcomes (baseline, 3 months and 6 months) of the study groups will be analysed using an analysis of variance. The level of significance will be set as p≤0.05.
Ethics and dissemination: The research ethics committee approved the study. It is expected to evaluate respiratory function in patients with ALS in the short, medium and long terms with home-based care protocol applied. The disease's rapid progression is a limitation for performing a long-term clinical study.
Trial registration number: RBR-3z23ts; Pre-results.
Keywords: clinical trials; motor neurone disease; neuromuscular disease; rehabilitation medicine.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Remote versus face-to-face home-based exercise programme in people with amyotrophic lateral sclerosis: protocol for a randomised clinical trial.BMJ Open. 2022 May 26;12(5):e056323. doi: 10.1136/bmjopen-2021-056323. BMJ Open. 2022. PMID: 35618326 Free PMC article.
-
Study protocol for a randomised, double-blind, placebo-controlled study evaluating the Efficacy of cannabis-based Medicine Extract in slowing the disease pRogression of Amyotrophic Lateral sclerosis or motor neurone Disease: the EMERALD trial.BMJ Open. 2019 Nov 11;9(11):e029449. doi: 10.1136/bmjopen-2019-029449. BMJ Open. 2019. PMID: 31719072 Free PMC article.
-
Correlation between Forced Vital Capacity and Slow Vital Capacity for the assessment of respiratory involvement in Amyotrophic Lateral Sclerosis: a prospective study.Amyotroph Lateral Scler Frontotemporal Degener. 2017 Feb;18(1-2):86-91. doi: 10.1080/21678421.2016.1249486. Epub 2016 Dec 4. Amyotroph Lateral Scler Frontotemporal Degener. 2017. PMID: 27915482
-
Respiratory muscle training in children and adults with neuromuscular disease.Cochrane Database Syst Rev. 2019 Sep 5;9(9):CD011711. doi: 10.1002/14651858.CD011711.pub2. Cochrane Database Syst Rev. 2019. PMID: 31487757 Free PMC article.
-
Amyotrophic lateral sclerosis: evaluation and treatment of respiratory impairment.Amyotroph Lateral Scler Other Motor Neuron Disord. 2002 Mar;3(1):5-13. doi: 10.1080/146608202317576480. Amyotroph Lateral Scler Other Motor Neuron Disord. 2002. PMID: 12061943 Review.
Cited by
-
Digital health solution for monitoring and surveillance of Amyotrophic Lateral Sclerosis in Brazil.Front Public Health. 2023 Aug 25;11:1209633. doi: 10.3389/fpubh.2023.1209633. eCollection 2023. Front Public Health. 2023. PMID: 37693725 Free PMC article.
References
-
- EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis:, Andersen PM, Abrahams S, et al. . EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS)--revised report of an EFNS task force. Eur J Neurol 2012;19:360–75. 10.1111/j.1468-1331.2011.03501.x - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
