Systemic immunity in cancer
- PMID: 33837297
- PMCID: PMC8034277
- DOI: 10.1038/s41568-021-00347-z
Systemic immunity in cancer
Abstract
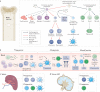
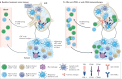
Immunotherapy has revolutionized cancer treatment, but efficacy remains limited in most clinical settings. Cancer is a systemic disease that induces many functional and compositional changes to the immune system as a whole. Immunity is regulated by interactions of diverse cell lineages across tissues. Therefore, an improved understanding of tumour immunology must assess the systemic immune landscape beyond the tumour microenvironment (TME). Importantly, the peripheral immune system is required to drive effective natural and therapeutically induced antitumour immune responses. In fact, emerging evidence suggests that immunotherapy drives new immune responses rather than the reinvigoration of pre-existing immune responses. However, new immune responses in individuals burdened with tumours are compromised even beyond the TME. Herein, we aim to comprehensively outline the current knowledge of systemic immunity in cancer.
Conflict of interest statement
M.H.S. is a co-founder and board member of Teiko Bio, receives research funding from Roche/Genentech, Bristol-Myers Squibb and Valitor, and has been a paid consultant for Earli, Five Prime Therapeutics, Ono Pharmaceutical and January Inc. B.M.A. is currently an employee of Teiko Bio. K.J.H.-G. declares no competing interests.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

