Applying evidence-based methods to the development and use of adverse outcome pathways
- PMID: 33837437
- PMCID: PMC9394185
- DOI: 10.14573/altex.2101211
Applying evidence-based methods to the development and use of adverse outcome pathways
Abstract
The workshop “Application of evidence-based methods to construct mechanistic frameworks for the development and use of non-animal toxicity tests” was organized by the Evidence-based Toxicology Collaboration and hosted by the Grading of Recommendations Assessment, Development and Evaluation Working Group on June 12, 2019. The purpose of the workshop was to bring together international regulatory bodies, risk assessors, academic scientists, and industry to explore how systematic review methods and the adverse outcome pathway framework could be combined to develop and use mechanistic test methods for predicting the toxicity of chemical substances in an evidence-based manner. The meeting covered the history of biological frameworks, the way adverse outcome pathways are currently developed, the basic principles of systematic methodology, including systematic reviews and evidence maps, and assessment of certainty in models, and adverse outcome pathways in particular. Specific topics were discussed via case studies in small break-out groups. The group concluded that adverse outcome pathways provide an important framework to support mechanism-based assessment in environmental health. The process of their development has a few challenges that could be addressed with systematic methods and automation tools. Addressing these challenges will increase the transparency of the evidence behind adverse outcome pathways and the consistency with which they are defined; this in turn will increase their value for supporting public health decisions. It was suggested to explore the details of applying systematic methods to adverse outcome pathway development in a series of case studies and workshops.
Keywords: evidence-based; non-animal method; systematic review; adverse outcome pathway; risk assessment.
Conflict of interest statement
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this meeting report.
Figures

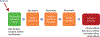
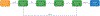
References
-
- EFSA (2010). Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA J 8, 1637. doi:10.2903/j.efsa.2010.1637 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

