Structure-guided paradigm shifts in flavivirus assembly and maturation mechanisms
- PMID: 33837721
- PMCID: PMC7510438
- DOI: 10.1016/bs.aivir.2020.08.003
Structure-guided paradigm shifts in flavivirus assembly and maturation mechanisms
Abstract
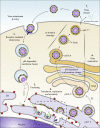
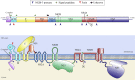
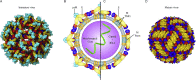
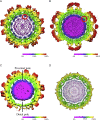
The flavivirus genus encompasses more than 75 unique viruses, including dengue virus which accounts for almost 390 million global infections annually. Flavivirus infection can result in a myriad of symptoms ranging from mild rash and flu-like symptoms, to severe encephalitis and even hemorrhagic fever. Efforts to combat the impact of these viruses have been hindered due to limited antiviral drug and vaccine development. However, the advancement of knowledge in the structural biology of flaviviruses over the last 25 years has produced unique perspectives for the identification of potential therapeutic targets. With particular emphasis on the assembly and maturation stages of the flavivirus life cycle, it is the goal of this review to comparatively analyze the structural similarities between flaviviruses to provide avenues for new research and innovation.
Keywords: Capsid; Dengue; Envelope; Flavivirus; Flavivirus assembly; Flavivirus maturation; Flavivirus structure; West Nile; Zika.
© 2020 Elsevier Inc. All rights reserved.
Figures





References
-
- Alsaleh K. The E glycoprotein plays an essential role in the high pathogenicity of 381 European -Mediterranean IS98 strain of West Nile virus. Virology. 2016;492:53–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

