Viral cell-to-cell spread: Conventional and non-conventional ways
- PMID: 33837723
- PMCID: PMC7522014
- DOI: 10.1016/bs.aivir.2020.09.002
Viral cell-to-cell spread: Conventional and non-conventional ways
Abstract
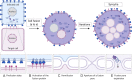
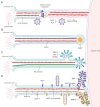
A critical step in the life cycle of a virus is spread to a new target cell, which generally involves the release of new viral particles from the infected cell which can then initiate infection in the next target cell. While cell-free viral particles released into the extracellular environment are necessary for long distance spread, there are disadvantages to this mechanism. These include the presence of immune system components, the low success rate of infection by single particles, and the relative fragility of viral particles in the environment. Several mechanisms of direct cell-to-cell spread have been reported for animal viruses which would avoid the issues associated with cell-free particles. A number of viruses can utilize several different mechanisms of direct cell-to-cell spread, but our understanding of the differential usage by these pathogens is modest. Although the mechanisms of cell-to-cell spread differ among viruses, there is a common exploitation of key pathways and components of the cellular cytoskeleton. Remarkably, some of the viral mechanisms of cell-to-cell spread are surprisingly similar to those used by bacteria. Here we summarize the current knowledge of the conventional and non-conventional mechanisms of viral spread, the common methods used to detect viral spread, and the impact that these mechanisms can have on viral pathogenesis.
Keywords: Cell-to-cell spread; Filopodia; Nanotubes; Syncytia; Virus.
© 2020 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Porcine Reproductive and Respiratory Syndrome Virus Utilizes Nanotubes for Intercellular Spread.J Virol. 2016 Apr 29;90(10):5163-5175. doi: 10.1128/JVI.00036-16. Print 2016 May 15. J Virol. 2016. PMID: 26984724 Free PMC article.
-
Electron tomography of the supramolecular structure of virus-infected cells.Curr Opin Struct Biol. 2010 Oct;20(5):632-9. doi: 10.1016/j.sbi.2010.08.007. Epub 2010 Sep 17. Curr Opin Struct Biol. 2010. PMID: 20850967 Review.
-
Viral interactions with the cytoskeleton: a hitchhiker's guide to the cell.Cell Microbiol. 2006 Mar;8(3):387-400. doi: 10.1111/j.1462-5822.2005.00679.x. Cell Microbiol. 2006. PMID: 16469052 Review.
-
Molecular mechanisms of virus spread and virion components as tools of virulence. A review.Acta Microbiol Immunol Hung. 2003;50(4):407-31. doi: 10.1556/AMicr.50.2003.4.8. Acta Microbiol Immunol Hung. 2003. PMID: 14750441 Review.
-
Viruses and apoptosis.Annu Rev Microbiol. 1999;53:577-628. doi: 10.1146/annurev.micro.53.1.577. Annu Rev Microbiol. 1999. PMID: 10547702 Review.
Cited by
-
Failure of Herpes Simplex Virus Glycoprotein D Antibodies to Elicit Antibody-Dependent Cell-Mediated Cytotoxicity: Implications for Future Vaccines.J Infect Dis. 2022 Nov 1;226(9):1489-1498. doi: 10.1093/infdis/jiac284. J Infect Dis. 2022. PMID: 35834278 Free PMC article.
-
Diversity of Intercellular Communication Modes: A Cancer Biology Perspective.Cells. 2024 Mar 12;13(6):495. doi: 10.3390/cells13060495. Cells. 2024. PMID: 38534339 Free PMC article. Review.
-
HIV-1 trans-Infection Mediated by DCs: The Tip of the Iceberg of Cell-to-Cell Viral Transmission.Pathogens. 2021 Dec 31;11(1):39. doi: 10.3390/pathogens11010039. Pathogens. 2021. PMID: 35055987 Free PMC article. Review.
-
Emerging Roles of the Unique Molecular Chaperone Cosmc in the Regulation of Health and Disease.Biomolecules. 2022 Nov 23;12(12):1732. doi: 10.3390/biom12121732. Biomolecules. 2022. PMID: 36551160 Free PMC article. Review.
-
Cell Fusion and Syncytium Formation in Betaherpesvirus Infection.Viruses. 2021 Sep 30;13(10):1973. doi: 10.3390/v13101973. Viruses. 2021. PMID: 34696402 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

