Weight gain before and after switch from TDF to TAF in a U.S. cohort study
- PMID: 33838004
- PMCID: PMC8035674
- DOI: 10.1002/jia2.25702
Weight gain before and after switch from TDF to TAF in a U.S. cohort study
Abstract
Introduction: Although weight gain has been reported with the use of integrase strand transfer inhibitors (InSTI), concurrent use of tenofovir alafenamide (TAF) has been implicated in recent studies. This study examined weight changes in people living with HIV (PLWH) who switched from tenofovir disoproxil fumarate (TDF) to TAF, to clarify the relative contribution to weight gain of core agents versus TDF to TAF switch.
Methods: Antiretroviral-experienced, virologically suppressed PLWH in the U.S. OPERA cohort were included if they switched from TDF to TAF (5NOV2015-28FEB2019) and either maintained all other antiretrovirals or switched from a non-InSTI to an InSTI. Linear mixed models were used to assess weight changes before/after the switch to TAF (restricted cubic splines on time) and rates of change over time (linear splines on time, based on the shape of the weight change curves). Changes in weight on TDF or TAF were assessed among those who maintained other antiretrovirals (overall, by core class), and those who maintained an InSTI or switched to an InSTI (by core agent). All models were adjusted for age, sex, race, (age-sex, race-sex interactions), BMI, CD4 cell count, endocrine disorders and concurrent medications that could affect weight.
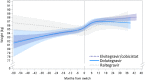
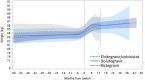
Results: A total of 6908 PLWH were included, with 5479 maintaining all other antiretrovirals (boosted protease inhibitor: 746, non-nucleoside reverse transcriptase inhibitor: 1452, InSTI: 3281) and 1429 switching from a non-InSTI to an InSTI (elvitegravir/cobicistat: 1120, dolutegravir: 174, bictegravir: 129). In adjusted models, modest weight gain was observed over time on TDF for most (0.24 to 0.71 kg/year); raltegravir was the exception with weight loss. Switching to TAF was associated with early, pronounced weight gain for all (1.80 to 4.47 kg/year). This effect with TAF switch was observed both in PLWH maintaining other antiretrovirals and those switching to an InSTI, regardless of which InSTI agent was used. Weight gain tended to slow down or plateau approximately nine months after switch to TAF.
Conclusions: In this large, diverse U.S. cohort of PLWH, switching from TDF to TAF was associated with pronounced weight gain immediately after switch, regardless of the core class or core agent, suggesting an independent effect of TAF on weight gain.
Keywords: Cohort; antiretroviral therapy; integrase strand transfer inhibitor; tenofovir alafenamide; tenofovir disoproxil fumarate; weight gain.
© 2021 Merck Sharp & Dohme Corp. Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Figures

References
-
- Pe SAX, Wohl D, Yin MT, Post F, DeJesus E, Saag M, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV‐1 infection: two randomised, double‐blind, phase 3, non‐inferiority trials. Lancet. 2015;385(9987):2606–15. - PubMed
-
- Mills A, Arribas JR, Andrade‐Villanueva J, DiPerri G, Van Lunzen J, Koenig E, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV‐1 infection: a randomised, active‐controlled, multicentre, open‐label, phase 3, non‐inferiority study. Lancet Infect Dis. 2016;16(1):43–52. - PubMed
-
- Gallant JE, Daar ES, Raffi F, Brinson C, Ruane P, DeJesus E, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed‐dose combinations containing emtricitabine as backbones for treatment of HIV‐1 infection in virologically suppressed adults: a randomised, double‐blind, active‐controlled phase 3 trial. Lancet HIV. 2016;3(4):e158–65. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

