Functional diversity of three tandem C-terminal carbohydrate-binding modules of a β-mannanase
- PMID: 33838183
- PMCID: PMC8121702
- DOI: 10.1016/j.jbc.2021.100638
Functional diversity of three tandem C-terminal carbohydrate-binding modules of a β-mannanase
Abstract
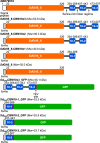
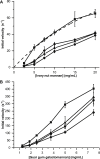
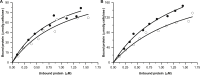
Carbohydrate active enzymes, such as those involved in plant cell wall and storage polysaccharide biosynthesis and deconstruction, often contain repeating noncatalytic carbohydrate-binding modules (CBMs) to compensate for low-affinity binding typical of protein-carbohydrate interactions. The bacterium Saccharophagus degradans produces an endo-β-mannanase of glycoside hydrolase family 5 subfamily 8 with three phylogenetically distinct family 10 CBMs located C-terminally from the catalytic domain (SdGH5_8-CBM10x3). However, the functional roles and cooperativity of these CBM domains in polysaccharide binding are not clear. To learn more, we studied the full-length enzyme, three stepwise CBM family 10 (CBM10) truncations, and GFP fusions of the individual CBM10s and all three domains together by pull-down assays, affinity gel electrophoresis, and activity assays. Only the C-terminal CBM10-3 was found to bind strongly to microcrystalline cellulose (dissociation constant, Kd = 1.48 μM). CBM10-3 and CBM10-2 bound galactomannan with similar affinity (Kd = 0.2-0.4 mg/ml), but CBM10-1 had 20-fold lower affinity for this substrate. CBM10 truncations barely affected specific activity on carob galactomannan and konjac glucomannan. Full-length SdGH5_8-CBM10x3 was twofold more active on the highly galactose-decorated viscous guar gum galactomannan and crystalline ivory nut mannan at high enzyme concentrations, but the specific activity was fourfold to ninefold reduced at low enzyme and substrate concentrations compared with the enzyme lacking CBM10-2 and CBM10-3. Comparison of activity and binding data for the different enzyme forms indicates unproductive and productive polysaccharide binding to occur. We conclude that the C-terminal-most CBM10-3 secures firm binding, with contribution from CBM10-2, which with CBM10-1 also provides spatial flexibility.
Keywords: GFP–domain fusion; affinity gel electrophoresis; enzyme kinetics; galactomannan; glucomannan; mannan; multimodular enzyme truncation; protein–carbohydrate interaction; pull-down assays; substrate specificity.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



Similar articles
-
Characterization of pH-tolerant and thermostable GH 134 β-1,4-mannanase SsGH134 possessing carbohydrate binding module 10 from Streptomyces sp. NRRL B-24484.J Biosci Bioeng. 2018 Mar;125(3):287-294. doi: 10.1016/j.jbiosc.2017.10.009. Epub 2017 Nov 16. J Biosci Bioeng. 2018. PMID: 29153955
-
The GH5 1,4-β-mannanase from Bifidobacterium animalis subsp. lactis Bl-04 possesses a low-affinity mannan-binding module and highlights the diversity of mannanolytic enzymes.BMC Biochem. 2015 Nov 11;16:26. doi: 10.1186/s12858-015-0055-4. BMC Biochem. 2015. PMID: 26558435 Free PMC article.
-
Expression and characterization of a Bifidobacterium adolescentis beta-mannanase carrying mannan-binding and cell association motifs.Appl Environ Microbiol. 2013 Jan;79(1):133-40. doi: 10.1128/AEM.02118-12. Epub 2012 Oct 12. Appl Environ Microbiol. 2013. PMID: 23064345 Free PMC article.
-
[Recent advances and prospect on structural biology of beta-mannanase--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1131-7. Wei Sheng Wu Xue Bao. 2009. PMID: 20030048 Review. Chinese.
-
Molecular advancements on over-expression, stability and catalytic aspects of endo-β-mannanases.Crit Rev Biotechnol. 2021 Feb;41(1):1-15. doi: 10.1080/07388551.2020.1825320. Epub 2020 Oct 9. Crit Rev Biotechnol. 2021. PMID: 33032458 Review.
Cited by
-
Whole genome sequence of Lactiplantibacillus plantarum MC5 and comparative analysis of eps gene clusters.Front Microbiol. 2023 May 2;14:1146566. doi: 10.3389/fmicb.2023.1146566. eCollection 2023. Front Microbiol. 2023. PMID: 37200914 Free PMC article.
-
Affinity Electrophoresis for Analysis of Catalytic Module-Carbohydrate Interactions.Methods Mol Biol. 2023;2657:91-101. doi: 10.1007/978-1-0716-3151-5_6. Methods Mol Biol. 2023. PMID: 37149524
-
Functional identification of two novel carbohydrate-binding modules of glucuronoxylanase CrXyl30 and their contribution to the lignocellulose saccharification.Biotechnol Biofuels Bioprod. 2023 Mar 8;16(1):40. doi: 10.1186/s13068-023-02290-7. Biotechnol Biofuels Bioprod. 2023. PMID: 36890582 Free PMC article.
-
Impact of Starch Binding Domain Fusion on Activities and Starch Product Structure of 4-α-Glucanotransferase.Molecules. 2023 Jan 30;28(3):1320. doi: 10.3390/molecules28031320. Molecules. 2023. PMID: 36770986 Free PMC article.
-
Time-resolved tracking of cellulose biosynthesis and assembly during cell wall regeneration in live Arabidopsis protoplasts.Sci Adv. 2025 Mar 21;11(12):eads6312. doi: 10.1126/sciadv.ads6312. Epub 2025 Mar 21. Sci Adv. 2025. PMID: 40117364 Free PMC article.
References
-
- Conway J.M., Crosby J.R., Hren A.P., Southerland R.T., Lee L.L., Lunin V.V., Alahuhta P., Himmel M.E., Bomble Y.J., Adams M.W.W., Kelly R.M. Novel multidomain, multifunctional glycoside hydrolases from highly lignocellulolytic Caldicellulosiruptor species. Aiche J. 2018;64:4218–4228.
-
- Ebringerová A. Structural diversity and application potential of hemicelluloses. Macromol. Symp. 2006;232:1–12.
-
- Dey P.M. Biochemistry of plant galactomannans. Adv. Carbohydr. Chem. Biochem. 1978;35:341–376.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

