Structure and Expression of Large (+)RNA Genomes of Viruses of Higher Eukaryotes
- PMID: 33838627
- PMCID: PMC7772802
- DOI: 10.1134/S0006297921030020
Structure and Expression of Large (+)RNA Genomes of Viruses of Higher Eukaryotes
Abstract
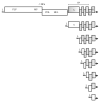
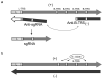
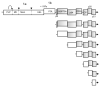
Viral positive-sense RNA genomes evolve rapidly due to the high mutation rates during replication and RNA recombination, which allowing the viruses to acquire and modify genes for their adaptation. The size of RNA genome is limited by several factors, including low fidelity of RNA polymerases and packaging constraints. However, the 12-kb size limit is exceeded in the two groups of eukaryotic (+)RNA viruses - animal nidoviruses and plant closteroviruses. These virus groups have several traits in common. Their genomes contain 5'-proximal genes that are expressed via ribosomal frameshifting and encode one or two papain-like protease domains, membrane-binding domain(s), methyltransferase, RNA helicase, and RNA polymerase. In addition, some nidoviruses (i.e., coronaviruses) contain replication-associated domains, such as proofreading exonuclease, putative primase, nucleotidyltransferase, and endonuclease. In both nidoviruses and closteroviruses, the 3'-terminal part of the genome contains genes for structural and accessory proteins expressed via a nested set of coterminal subgenomic RNAs. Coronaviruses and closteroviruses have evolved to form flexuous helically symmetrical nucleocapsids as a mean to resolve packaging constraints. Since phylogenetic reconstructions of the RNA polymerase domains indicate only a marginal relationship between the nidoviruses and closteroviruses, their similar properties likely have evolved convergently, along with the increase in the genome size.
Keywords: SARS-CoV; closteroviruses; evolution; gene expression; nidoviruses; viral positive-sense RNA genomes.
Conflict of interest statement
The author declares no conflict of interest. This article does not contain description of studies with the involvement of humans or animal subjects.
Figures
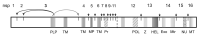
References
-
- Dolja V. V., Karasev A. V., Koonin E. V. Molecular biology and evolution of closteroviruses: sophisticated build-up of large RNA genomes. Annu. Rev. Phytopathol. 1994;32:261–285. doi: 10.1146/annurev.py.32.090194.001401. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

