Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial
- PMID: 33838657
- PMCID: PMC8035859
- DOI: 10.1186/s12879-021-06045-3
Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial
Erratum in
-
Correction to: Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial.BMC Infect Dis. 2021 May 11;21(1):436. doi: 10.1186/s12879-021-06130-7. BMC Infect Dis. 2021. PMID: 33975548 Free PMC article. No abstract available.
Abstract
Background: Although almost a year has passed since the Coronavirus disease 2019 (COVID-19) outbreak and promising reports of vaccines have been presented, we still have a long way until these measures are available for all. Furthermore, the most appropriate corticosteroid and dose in the treatment of COVID-19 have remained uncertain. We conducted a study to assess the effectiveness of methylprednisolone treatment versus dexamethasone for hospitalized COVID-19 patients.
Methods: In this prospective triple-blinded randomized controlled trial, we enrolled 86 hospitalized COVID-19 patients from August to November 2020, in Shiraz, Iran. The patients were randomly allocated into two groups to receive either methylprednisolone (2 mg/kg/day; intervention group) or dexamethasone (6 mg/day; control group). Data were assessed based on a 9-point WHO ordinal scale extending from uninfected (point 0) to death (point 8).
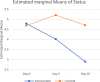
Results: There were no significant differences between the groups on admission. However, the intervention group demonstrated significantly better clinical status compared to the control group at day 5 (4.02 vs. 5.21, p = 0.002) and day 10 (2.90 vs. 4.71, p = 0.001) of admission. There was also a significant difference in the overall mean score between the intervention group and the control group, (3.909 vs. 4.873 respectively, p = 0.004). The mean length of hospital stay was 7.43 ± 3.64 and 10.52 ± 5.47 days in the intervention and control groups, respectively (p = 0.015). The need for a ventilator was significantly lower in the intervention group than in the control group (18.2% vs 38.1% p = 0.040).
Conclusion: In hospitalized hypoxic COVID-19 patients, methylprednisolone demonstrated better results compared to dexamethasone.
Trial registration: The trial was registered with IRCT.IR (08/04/2020-No. IRCT20200204046369N1 ).
Keywords: COVID-19; Corticosteroid; Dexamethasone; Methylprednisolone; Randomized controlled trial.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures
Comment in
-
Therapeutic Application of Corticosteroids in COVID-19: A Focus on Optimum Dose and Duration of Therapy.J Clin Pharmacol. 2021 Sep;61(9):1145-1148. doi: 10.1002/jcph.1929. Epub 2021 Jul 10. J Clin Pharmacol. 2021. PMID: 34157144 Free PMC article. No abstract available.
References
-
- World Health Organization. WHO announces COVID-19 outbreak a pandemic, 2020. Available from: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus.... Accessed 7 December 2020.
-
- World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). 2020. Available from: https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting.... Accessed 11 December 2020.
-
- World Health Organization. Clinical management of severe acute respiratory infection when Middle East respiratory syndrome coronavirus ( MERS-CoV) infection is suspected: interim guidance. 2019. Available from: https://apps.who.int/iris/handle/10665/178529. Accessed 3 January 2021.
-
- Worldometer. COVID-19 Coronavirus Pandemic. 2020. Available from: https://www.worldometers.info/. Accessed 15 January 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources