Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways
- PMID: 33838688
- PMCID: PMC8035743
- DOI: 10.1186/s13046-021-01915-9
Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways
Erratum in
-
Correction: Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways.J Exp Clin Cancer Res. 2023 Jun 24;42(1):153. doi: 10.1186/s13046-023-02733-x. J Exp Clin Cancer Res. 2023. PMID: 37355608 Free PMC article. No abstract available.
Abstract
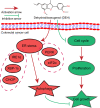
Background: Dehydrodiisoeugenol (DEH), a novel lignan component extracted from nutmeg, which is the seed of Myristica fragrans Houtt, displays noticeable anti-inflammatory and anti-allergic effects in digestive system diseases. However, the mechanism of its anticancer activity in gastrointestinal cancer remains to be investigated.
Methods: In this study, the anticancer effect of DEH on human colorectal cancer and its underlying mechanism were evaluated. Assays including MTT, EdU, Plate clone formation, Soft agar, Flow cytometry, Electron microscopy, Immunofluorescence and Western blotting were used in vitro. The CDX and PDX tumor xenograft models were used in vivo.
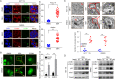
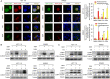
Results: Our findings indicated that treatment with DEH arrested the cell cycle of colorectal cancer cells at the G1/S phase, leading to significant inhibition in cell growth. Moreover, DEH induced strong cellular autophagy, which could be inhibited through autophagic inhibitors, with a rction in the DEH-induced inhibition of cell growth in colorectal cancer cells. Further analysis indicated that DEH also induced endoplasmic reticulum (ER) stress and subsequently stimulated autophagy through the activation of PERK/eIF2α and IRE1α/XBP-1 s/CHOP pathways. Knockdown of PERK or IRE1α significantly decreased DEH-induced autophagy and retrieved cell viability in cells treated with DEH. Furthermore, DEH also exhibited significant anticancer activities in the CDX- and PDX-models.
Conclusions: Collectively, our studies strongly suggest that DEH might be a potential anticancer agent against colorectal cancer by activating ER stress-induced inhibition of autophagy.
Keywords: Anticancer agent; Autophagy inhibition; Colorectal cancer; Dehydrodiisoeugenol (DEH); Endoplasmic reticulum (ER) stress.
Conflict of interest statement
The corresponding author and all the co-authors have agreed to the publication of the manuscript to Journal of Experimental and Clinical Cancer Research as a research article and declare that they have no conflict of interest as to the results presented.
The authors declare that they have no competing interests.
Figures





Similar articles
-
Dehydrodiisoeugenol targets NOD2 exerting dual effects against colitis and colorectal cancer: a double-edged sword.Mol Med. 2025 Jun 5;31(1):221. doi: 10.1186/s10020-025-01193-7. Mol Med. 2025. PMID: 40468178 Free PMC article.
-
A Marine-Derived Small Molecule Inhibits Prostate Cancer Growth by Promoting Endoplasmic Reticulum Stress Induced Apoptosis and Autophagy.Phytother Res. 2024 Dec;38(12):6004-6022. doi: 10.1002/ptr.8354. Epub 2024 Oct 30. Phytother Res. 2024. PMID: 39474779
-
[Dehydrodiisoeugenol resists H1N1 virus infection via TFEB/autophagy-lysosome pathway].Zhongguo Zhong Yao Za Zhi. 2025 Mar;50(6):1650-1658. doi: 10.19540/j.cnki.cjcmm.20241107.701. Zhongguo Zhong Yao Za Zhi. 2025. PMID: 40350952 Chinese.
-
Sodium Butyrate Induces Endoplasmic Reticulum Stress and Autophagy in Colorectal Cells: Implications for Apoptosis.PLoS One. 2016 Jan 19;11(1):e0147218. doi: 10.1371/journal.pone.0147218. eCollection 2016. PLoS One. 2016. PMID: 26784903 Free PMC article.
-
Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate.Cell Death Dis. 2015 Jul 30;6(7):e1822. doi: 10.1038/cddis.2015.183. Cell Death Dis. 2015. PMID: 26225772 Free PMC article. Review.
Cited by
-
Combined bulk RNA-seq and single-cell RNA-seq identifies a necroptosis-related prognostic signature associated with inhibitory immune microenvironment in glioma.Front Immunol. 2022 Nov 17;13:1013094. doi: 10.3389/fimmu.2022.1013094. eCollection 2022. Front Immunol. 2022. PMID: 36466844 Free PMC article.
-
Endoplasmic reticulum stress-a key guardian in cancer.Cell Death Discov. 2024 Jul 30;10(1):343. doi: 10.1038/s41420-024-02110-3. Cell Death Discov. 2024. PMID: 39080273 Free PMC article. Review.
-
GC-MS, LC-MS, and network pharmacology analysis to investigate the chemical profiles and potential pharmacological activities in flower buds and flowers of Lonicera japonica Thunb.PLoS One. 2025 Apr 23;20(4):e0320293. doi: 10.1371/journal.pone.0320293. eCollection 2025. PLoS One. 2025. PMID: 40267096 Free PMC article.
-
The Underlying Roles of Exosome-Associated PIGR in Fatty Acid Metabolism and Immune Signaling in Colorectal Cancer.J Oncol. 2022 Sep 15;2022:4675683. doi: 10.1155/2022/4675683. eCollection 2022. J Oncol. 2022. PMID: 36157233 Free PMC article.
-
Cortex Mori extracts induce apoptosis and inhibit tumor invasion via blockage of the PI3K/AKT signaling in melanoma cells.Front Pharmacol. 2022 Oct 19;13:1007279. doi: 10.3389/fphar.2022.1007279. eCollection 2022. Front Pharmacol. 2022. PMID: 36339598 Free PMC article.
References
-
- Shimodaira Y, Takahashi S, Kinouchi Y, Endo K, Shiga H, Kakuta Y, Kuroha M, Shimosegawa T. Modulation of endoplasmic reticulum (ER) stress-induced autophagy by C/EBP homologous protein (CHOP) and inositol-requiring enzyme 1α (IRE1α) in human colon cancer cells. Biochem Biophys Res Commun. 2014;445(2):524–533. doi: 10.1016/j.bbrc.2014.02.054. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 31672496/the National Natural Science Foundation of China
- 31802142/the National Natural Science Foundation of China
- 81872071/the National Natural Science Foundation of China
- 81672502/the National Natural Science Foundation of China
- 2016YFC1302204/the National Key Research and Development Program of China
- 2017YFC1308601/the National Key Research and Development Program of China
- cstc2019jcyj-zdxmX0033/Chongqing Natural Science foundation of Chongqing
- CXTDX201601010/Chongqing University Innovation Team Building Program funded projects
- XDJK2019C089/the Fundamental Research Funds for the Central Universities
- 2019T120801/the China Postdoctoral Science Foundation
- 2017M620408/the China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

