The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial
- PMID: 33838758
- PMCID: PMC8047813
- DOI: 10.1016/S0140-6736(21)00246-4
The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial
Erratum in
-
Department of Error.Lancet. 2021 Apr 24;397(10284):1544. doi: 10.1016/S0140-6736(21)00886-2. Lancet. 2021. PMID: 33894831 Free PMC article. No abstract available.
-
Department of Error.Lancet. 2021 May 15;397(10287):1808. doi: 10.1016/S0140-6736(21)01011-4. Lancet. 2021. PMID: 33992147 Free PMC article. No abstract available.
Abstract
Background: Valproate is a first-line treatment for patients with newly diagnosed idiopathic generalised or difficult to classify epilepsy, but not for women of child-bearing potential because of teratogenicity. Levetiracetam is increasingly prescribed for these patient populations despite scarcity of evidence of clinical effectiveness or cost-effectiveness. We aimed to compare the long-term clinical effectiveness and cost-effectiveness of levetiracetam compared with valproate in participants with newly diagnosed generalised or unclassifiable epilepsy.
Methods: We did an open-label, randomised controlled trial to compare levetiracetam with valproate as first-line treatment for patients with generalised or unclassified epilepsy. Adult and paediatric neurology services (69 centres overall) across the UK recruited participants aged 5 years or older (with no upper age limit) with two or more unprovoked generalised or unclassifiable seizures. Participants were randomly allocated (1:1) to receive either levetiracetam or valproate, using a minimisation programme with a random element utilising factors. Participants and investigators were aware of treatment allocation. For participants aged 12 years or older, the initial advised maintenance doses were 500 mg twice per day for levetiracetam and valproate, and for children aged 5-12 years, the initial daily maintenance doses advised were 25 mg/kg for valproate and 40 mg/kg for levetiracetam. All drugs were administered orally. SANAD II was designed to assess the non-inferiority of levetiracetam compared with valproate for the primary outcome time to 12-month remission. The non-inferiority limit was a hazard ratio (HR) of 1·314, which equates to an absolute difference of 10%. A HR greater than 1 indicated that an event was more likely on valproate. All participants were included in the intention-to-treat (ITT) analysis. Per-protocol (PP) analyses excluded participants with major protocol deviations and those who were subsequently diagnosed as not having epilepsy. Safety analyses included all participants who received one dose of any study drug. This trial is registered with the ISRCTN registry, 30294119 (EudraCt number: 2012-001884-64).
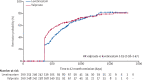
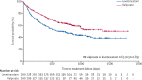
Findings: 520 participants were recruited between April 30, 2013, and Aug 2, 2016, and followed up for a further 2 years. 260 participants were randomly allocated to receive levetiracetam and 260 participants to receive valproate. The ITT analysis included all participants and the PP analysis included 255 participants randomly allocated to valproate and 254 randomly allocated to levetiracetam. Median age of participants was 13·9 years (range 5·0-94·4), 65% were male and 35% were female, 397 participants had generalised epilepsy, and 123 unclassified epilepsy. Levetiracetam did not meet the criteria for non-inferiority in the ITT analysis of time to 12-month remission (HR 1·19 [95% CI 0·96-1·47]); non-inferiority margin 1·314. The PP analysis showed that the 12-month remission was superior with valproate than with levetiracetam. There were two deaths, one in each group, that were unrelated to trial treatments. Adverse reactions were reported by 96 (37%) participants randomly assigned to valproate and 107 (42%) participants randomly assigned to levetiracetam. Levetiracetam was dominated by valproate in the cost-utility analysis, with a negative incremental net health benefit of -0·040 (95% central range -0·175 to 0·037) and a probability of 0·17 of being cost-effectiveness at a threshold of £20 000 per quality-adjusted life-year. Cost-effectiveness was based on differences between treatment groups in costs and quality-adjusted life-years.
Interpretation: Compared with valproate, levetiracetam was found to be neither clinically effective nor cost-effective. For girls and women of child-bearing potential, these results inform discussions about benefit and harm of avoiding valproate.
Funding: National Institute for Health Research Health Technology Assessment Programme.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests AM reports grants from the National Institute for Health Research Health Technology Assessment, during the conduct of the study, as well as grants from UCB Pharma, outside of the submitted work. JPL reports grants from University of Liverpool during the conduct of the study; grants and personal fees from UCB Pharma; and personal fees from Eisai, Janssen CIlag Pharmaceuticals, GW Pharmaceuticals, GSK Pharma, outside of the submitted work. GS reports personal fees from UCB Pharma, Eisai, Arvelle Therapeutics GmbH, outside of the submitted work. CP reports grants from National Institute for Health Research Health Technology Assessment Programme during the conduct of this study. CT reports grants from the National Institute for Health Research, during the conduct of the study. DH reports grants from National Institute for Health Research Health Technology Assessment Programme during the conduct of the study. RM reports personal fees from UCB Pharma and grants from UCB Pharma and Sanofi, outside of the submitted work. PES is a member of the NICE Panel for Epilepsy guideline 2021 and is an editor of the journal Practical Neurology. All other authors declare no competing interests.
Figures
Comment in
-
Newer versus older antiseizure medications: further forward?Lancet. 2021 Apr 10;397(10282):1327-1329. doi: 10.1016/S0140-6736(21)00435-9. Lancet. 2021. PMID: 33838747 No abstract available.
-
Epileptic Seizures in Alzheimer's Disease: What Are the Implications of SANAD II?J Alzheimers Dis. 2022;85(2):527-529. doi: 10.3233/JAD-215154. J Alzheimers Dis. 2022. PMID: 34842191
References
-
- Hauser WAHD. Demos Medical Pub; New York: 1990. Epilepsy: frequency, causes and consequences.
-
- Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the commission on classification and terminology of the international league against epilepsy. Epilepsia. 1981;22:489–501. No author. - PubMed
-
- Proposal for revised classification of epilepsies and epileptic syndromes. commission on classification and terminology of the international league against epilepsy. Epilepsia. 1989;30:389–399. No author. - PubMed
-
- Holland P, Lane S, Whitehead M, Marson AG, Jacoby A. Labor market participation following onset of seizures and early epilepsy: findings from a UK cohort. Epilepsia. 2009;50:1030–1039. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

