Altered sacral neural crest development in Pax3 spina bifida mutants underlies deficits of bladder innervation and function
- PMID: 33839113
- PMCID: PMC8172446
- DOI: 10.1016/j.ydbio.2021.03.024
Altered sacral neural crest development in Pax3 spina bifida mutants underlies deficits of bladder innervation and function
Abstract
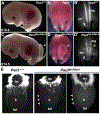
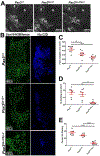
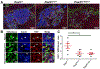
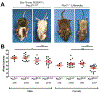
Mouse models of Spina bifida (SB) have been instrumental for identifying genes, developmental processes, and environmental factors that influence neurulation and neural tube closure. Beyond the prominent neural tube defects, other aspects of the nervous system can be affected in SB with significant changes in essential bodily functions such as urination. SB patients frequently experience bladder dysfunction and SB fetuses exhibit reduced density of bladder nerves and smooth muscle although the developmental origins of these deficits have not been determined. The Pax3 Splotch-delayed (Pax3Sp-d) mouse model of SB is one of a very few mouse SB models that survives to late stages of gestation. Through analysis of Pax3Sp-d mutants we sought to define how altered bladder innervation in SB might arise by tracing sacral neural crest (NC) development, pelvic ganglia neuronal differentiation, and assessing bladder nerve fiber density. In Pax3Sp-d/Sp-d fetal mice we observed delayed migration of Sox10+ NC-derived progenitors (NCPs), deficient pelvic ganglia neurogenesis, and reduced density of bladder wall innervation. We further combined NC-specific deletion of Pax3 with the constitutive Pax3Sp-d allele in an effort to generate viable Pax3 mutants to examine later stages of bladder innervation and postnatal bladder function. Neural crest specific deletion of a Pax3 flox allele, using a Sox10-cre driver, in combination with a constitutive Pax3Sp-d mutation produced postnatal viable offspring that exhibited altered bladder function as well as reduced bladder wall innervation and altered connectivity between accessory ganglia at the bladder neck. Combined, the results show that Pax3 plays critical roles within sacral NC that are essential for initiation of neurogenesis and differentiation of autonomic neurons within pelvic ganglia.
Keywords: Autonomic nervous system; Bladder; Lower urinary tract; Pax3; Pelvic ganglia; Sacral neural crest.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare no competing or financial interests.
Figures






Similar articles
-
Transgenic rescue of congenital heart disease and spina bifida in Splotch mice.Development. 1999 Jun;126(11):2495-503. doi: 10.1242/dev.126.11.2495. Development. 1999. PMID: 10226008
-
Migration pathways of sacral neural crest during development of lower urogenital tract innervation.Dev Biol. 2017 Sep 1;429(1):356-369. doi: 10.1016/j.ydbio.2017.04.011. Epub 2017 Apr 25. Dev Biol. 2017. PMID: 28449850 Free PMC article.
-
Genetic interaction of Pax3 mutation and canonical Wnt signaling modulates neural tube defects and neural crest abnormalities.Genesis. 2021 Nov;59(11):e23445. doi: 10.1002/dvg.23445. Epub 2021 Sep 7. Genesis. 2021. PMID: 34490995
-
Understanding the causes and prevention of neural tube defects: Insights from the splotch mouse model.Birth Defects Res A Clin Mol Teratol. 2009 Apr;85(4):322-30. doi: 10.1002/bdra.20539. Birth Defects Res A Clin Mol Teratol. 2009. PMID: 19180568 Review.
-
Mini-review: toward understanding mechanisms of genetic neural tube defects in mice.Teratology. 1999 Nov;60(5):292-305. doi: 10.1002/(SICI)1096-9926(199911)60:5<292::AID-TERA10>3.0.CO;2-6. Teratology. 1999. PMID: 10525207 Review.
Cited by
-
Innervation in organogenesis.Curr Top Dev Biol. 2022;148:195-235. doi: 10.1016/bs.ctdb.2022.02.004. Epub 2022 Mar 12. Curr Top Dev Biol. 2022. PMID: 35461566 Free PMC article.
-
Single-cell profiling coupled with lineage analysis reveals vagal and sacral neural crest contributions to the developing enteric nervous system.Elife. 2023 Oct 25;12:e79156. doi: 10.7554/eLife.79156. Elife. 2023. PMID: 37877560 Free PMC article.
References
-
- Anderson RB, Stewart AL, Young HM, 2006. Phenotypes of neural-crest-derived cells in vagal and sacral pathways. Cell Tissue Res 323, 11–25. - PubMed
-
- Arellano J, Xelhuantzi N, Mirto N, Hernandez ME, Cruz Y, 2019. Neural interrelationships of autonomic ganglia from the pelvic region of male rats. Auton Neurosci 217, 26–34. - PubMed
-
- Barber TD, Barber MC, Tomescu O, Barr FG, Ruben S, Friedman TB, 2002. Identification of target genes regulated by PAX3 and PAX3-FKHR in embryogenesis and alveolar rhabdomyosarcoma. Genomics 79, 278–284. - PubMed
-
- Borycki AG, Li J, Jin F, Emerson CP, Epstein JA, 1999. Pax3 functions in cell survival and in pax7 regulation. Development 126, 1665–1674. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

