Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET
- PMID: 33839159
- PMCID: PMC8113745
- DOI: 10.1016/j.jbc.2021.100641
Discovery of amivantamab (JNJ-61186372), a bispecific antibody targeting EGFR and MET
Abstract
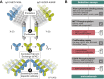
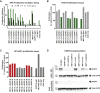
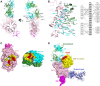
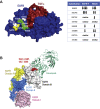
A bispecific antibody (BsAb) targeting the epidermal growth factor receptor (EGFR) and mesenchymal-epithelial transition factor (MET) pathways represents a novel approach to overcome resistance to targeted therapies in patients with non-small cell lung cancer. In this study, we sequentially screened a panel of BsAbs in a combinatorial approach to select the optimal bispecific molecule. The BsAbs were derived from different EGFR and MET parental monoclonal antibodies. Initially, molecules were screened for EGFR and MET binding on tumor cell lines and lack of agonistic activity toward MET. Hits were identified and further screened based on their potential to induce untoward cell proliferation and cross-phosphorylation of EGFR by MET via receptor colocalization in the absence of ligand. After the final step, we selected the EGFR and MET arms for the lead BsAb and added low fucose Fc engineering to generate amivantamab (JNJ-61186372). The crystal structure of the anti-MET Fab of amivantamab bound to MET was solved, and the interaction between the two molecules in atomic details was elucidated. Amivantamab antagonized the hepatocyte growth factor (HGF)-induced signaling by binding to MET Sema domain and thereby blocking HGF β-chain-Sema engagement. The amivantamab EGFR epitope was mapped to EGFR domain III and residues K443, K465, I467, and S468. Furthermore, amivantamab showed superior antitumor activity over small molecule EGFR and MET inhibitors in the HCC827-HGF in vivo model. Based on its unique mode of action, amivantamab may provide benefit to patients with malignancies associated with aberrant EGFR and MET signaling.
Keywords: EGFR exon 20 insertion; MET amplification; amivantamab; bispecific anti-EGFR×MET antibody; combinatorial screening; crystal structure; epitope mapping; non–small cell lung cancer (NSCLC).
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest Joost Neijssen, Luus Wiegman, and Janine Schuurman are employees of Genmab. Paul Parren was an employee of Genmab when this work was completed. Rosa M.F. Cardoso, Kristen Chevalier, and Sheri Moores are employees of Janssen Research & Development. Mark Chiu, Mark Anderson, and William Strohl were employees of Janssen Research & Development when this work was completed.
Figures


References
-
- Birchmeier C., Birchmeier W., Gherardi E., Vande Woude G.F. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 2003;4:915–925. - PubMed
-
- Liu X., Yao W., Newton R.C., Scherle P.A. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin. Investig. Drugs. 2008;17:997–1011. - PubMed
-
- Bean J., Brennan C., Shih J.Y., Riely G., Viale A., Wang L., Chitale D., Motoi N., Szoke J., Broderick S., Balak M., Chang W.C., Yu C.J., Gazdar A., Pass H. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20932–20937. - PMC - PubMed
-
- Arrieta O., Cardona A.F., Martin C., Mas-Lopez L., Corrales-Rodriguez L., Bramuglia G., Castillo-Fernandez O., Meyerson M., Amieva-Rivera E., Campos-Parra A.D., Carranza H., Gomez de la Torre J.C., Powazniak Y., Aldaco-Sarvide F., Vargas C. Updated frequency of EGFR and KRAS mutations in NonSmall-cell lung cancer in Latin America: The Latin-American Consortium for the investigation of lung cancer (CLICaP) J. Thorac. Oncol. 2015;10:838–843. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

