Enhanced throughput of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time RT-PCR panel by assay multiplexing and specimen pooling
- PMID: 33839185
- PMCID: PMC8028606
- DOI: 10.1016/j.jviromet.2021.114149
Enhanced throughput of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) real-time RT-PCR panel by assay multiplexing and specimen pooling
Abstract
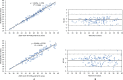
A multiplex real-time reverse transcriptase-polymerase chain reaction (rRT-PCR) assay for detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was developed based on the same primer and probe sequences of an existing U.S. CDC Emergency Use authorized test panel, targeting SARS-CoV-2 N1, N2 and human RNase P genes in singleplex. Both singleplex and multiplex assays demonstrated linear dynamic ranges of 8 orders of magnitude and analytical limits of detection of 5 RNA transcript copies/reaction. Both assays showed 100 % agreement with 364 previously characterized clinical specimens (146 positive and 218 negative) for detection of SARS-CoV-2 RNA. To further increase testing throughput, 40 positive and 20 negative four-specimen pools were tested by the multiplex assay and showed 97.75 % and 100 % congruence with individual specimen tests, respectively. rRT-PCR assay multiplexing and sample pooling, individually or in combination, can substantially increase throughput of SARS-CoV-2 testing.
Keywords: COVID-19; Coronavirus; Multiplex; Pooling; Real-time RT-PCR; SARS-CoV-2.
Published by Elsevier B.V.
Conflict of interest statement
The authors report no declarations of interest.
Figures


References
-
- A Shiny app for pooled testing. 2020 Available online: https://www.chrisbilder.com/shiny. (accessed on 01/12/2020).
-
- Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. - PubMed
-
- Centers for Disease Control and Prevention . 2020. CDC 2019-novel Coronavirus (2019-nCoV) Real-time RT-PCR Diagnostic Panel.https://www.fda.gov/media/134922/download
-
- Centers for Disease Control and Prevention . 2020. Interim Guidance for Use of Pooling Procedures in SARS-CoV-2 Diagnostic, Screening, and Surveillance Testing.https://www.cdc.gov/coronavirus/2019-ncov/lab/pooling-procedures.html
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

