Exercise Training Improves Tumor Control by Increasing CD8+ T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade
- PMID: 33839688
- PMCID: PMC8295193
- DOI: 10.1158/2326-6066.CIR-20-0499
Exercise Training Improves Tumor Control by Increasing CD8+ T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade
Abstract
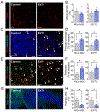
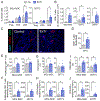
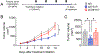
The mechanisms behind the antitumor effects of exercise training (ExTr) are not fully understood. Using mouse models of established breast cancer, we examined here the causal role of CD8+ T cells in the benefit acquired from ExTr in tumor control, as well as the ability of ExTr to improve immunotherapy responses. We implanted E0771, EMT6, MMTV-PyMT, and MCa-M3C breast cancer cells orthotopically in wild-type or Cxcr3-/- female mice and initiated intensity-controlled ExTr sessions when tumors reached approximately 100 mm3 We characterized the tumor microenvironment (TME) using flow cytometry, transcriptome analysis, proteome array, ELISA, and immunohistochemistry. We used antibodies against CD8+ T cells for cell depletion. Treatment with immune checkpoint blockade (ICB) consisted of anti-PD-1 alone or in combination with anti-CTLA-4. ExTr delayed tumor growth and induced vessel normalization, demonstrated by increased pericyte coverage and perfusion and by decreased hypoxia. ExTr boosted CD8+ T-cell infiltration, with enhanced effector function. CD8+ T-cell depletion prevented the antitumor effect of ExTr. The recruitment of CD8+ T cells and the antitumor effects of ExTr were abrogated in Cxcr3-/- mice, supporting the causal role of the CXCL9/CXCL11-CXCR3 pathway. ExTr also sensitized ICB-refractory breast cancers to treatment. Our results indicate that ExTr can normalize the tumor vasculature, reprogram the immune TME, and enhance the antitumor activity mediated by CD8+ T cells via CXCR3, boosting ICB responses. Our findings and mechanistic insights provide a rationale for the clinical translation of ExTr to improve immunotherapy of breast cancer.
©2021 American Association for Cancer Research.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

