Single-cell dual-omics reveals the transcriptomic and epigenomic diversity of cardiac non-myocytes
- PMID: 33839759
- PMCID: PMC9074971
- DOI: 10.1093/cvr/cvab134
Single-cell dual-omics reveals the transcriptomic and epigenomic diversity of cardiac non-myocytes
Abstract
Aims: The precise cellular identity and molecular features of non-myocytes (non-CMs) in a mammalian heart at a single-cell level remain elusive. Depiction of epigenetic landscape with transcriptomic signatures using the latest single-cell multi-omics has the potential to unravel the molecular programs underlying the cellular diversity of cardiac non-myocytes. Here, we characterized the molecular and cellular features of cardiac non-CM populations in the adult murine heart at the single-cell level.
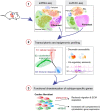
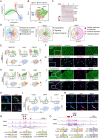
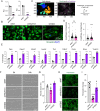
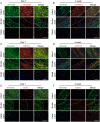
Methods and results: Through single-cell dual omics analysis, we mapped the epigenetic landscapes, characterized the transcriptomic profiles and delineated the molecular signatures of cardiac non-CMs in the adult murine heart. Distinct cis-regulatory elements and trans-acting factors for the individual major non-CM cell types (endothelial cells, fibroblast, pericytes, and immune cells) were identified. In particular, unbiased sub-clustering and functional annotation of cardiac fibroblasts (FBs) revealed extensive FB heterogeneity and identified FB sub-types with functional states related to the cellular response to stimuli, cytoskeleton organization, and immune regulation, respectively. We further explored the function of marker genes Hsd11b1 and Gfpt2 that label major FB subpopulations and determined the distribution of Hsd11b1+ and Gfp2+ FBs in murine healthy and diseased hearts.
Conclusions: In summary, we characterized the non-CM cellular identity at the transcriptome and epigenome levels using single-cell omics approaches and discovered previously unrecognized cardiac fibroblast subpopulations with unique functional states.
Keywords: Fibroblast; Murine adult heart; Non-myocytes; Single-cell ATAC-seq; Single-cell transcriptomics.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures




Comment in
-
Cardiac fibroblast heterogeneity and dynamics through the lens of single-cell dual 'omics.Cardiovasc Res. 2022 May 6;118(6):1380-1382. doi: 10.1093/cvr/cvac037. Cardiovasc Res. 2022. PMID: 35425951 No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

