Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study
- PMID: 33839790
- PMCID: PMC8830055
- DOI: 10.1210/clinem/dgab232
Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study
Abstract
Context: Clinical practice guidelines (CPGs) are key instruments to implement the practice of evidence-based medicine. We aimed to evaluate the methodological quality and variations in CPGs recommendations on the diagnosis and management of polycystic ovary syndrome (PCOS).
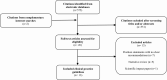
Evidence acquisition: We searched MEDLINE, EMBASE, and CENTRAL until December 2020 for all evidence-based CPGs and consensus statements on PCOS. We extracted data in duplicate to map clinical recommendations across prespecified disease domains and assessed CPGs methodological quality of using the Appraisal of Guidelines, Research & Evaluation II tool.
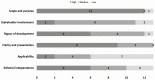
Evidence synthesis: We included 13 PCOS CPGs published between 2007 and 2018. CPGs recommendations were mostly focused on screening for and managing metabolic disease (12/13, 92%), followed by cardiovascular risk assessment (10/13, 77%). Mental health (8/13, 62%) and diagnosis in adolescents (7/13, 54%) were the least reported domains. Most CPGs had a high quality for scope and purpose description (12/13, 92%) while stakeholder's involvement and applicability of recommendations to clinical practice were appropriate in only 2 CPGs (2/13, 15%). We identified inconsistency in recommendations on PCOS diagnosis in adolescents, optimal lifestyle interventions, hirsutism and acne treatments, interventions to reduce the risk of ovarian hyperstimulation syndrome, the frequency and screening criteria for metabolic and cardiovascular disease, and optimal screening tools for mental health illness in women with PCOS.
Conclusion: Current CPGs on the diagnosis and management of PCOS vary in their scope and methodological quality, which may hinder evidence translation into clinical practice. We identified disease domains with existing evidence gap to guide future research and guideline updates.
Keywords: AGREE tool; clinical CPGs; polycystic ovary syndrome; quality; systematic review.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
Comment in
-
Letter to the Editor from Teede: "Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study".J Clin Endocrinol Metab. 2022 Feb 17;107(3):e1321-e1322. doi: 10.1210/clinem/dgab656. J Clin Endocrinol Metab. 2022. PMID: 34679180 No abstract available.
Similar articles
-
Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.Fertil Steril. 2018 Aug;110(3):364-379. doi: 10.1016/j.fertnstert.2018.05.004. Epub 2018 Jul 19. Fertil Steril. 2018. PMID: 30033227 Free PMC article. Review.
-
Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome.Hum Reprod. 2018 Sep 1;33(9):1602-1618. doi: 10.1093/humrep/dey256. Hum Reprod. 2018. PMID: 30052961 Free PMC article.
-
Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome.J Clin Endocrinol Metab. 2023 Sep 18;108(10):2447-2469. doi: 10.1210/clinem/dgad463. J Clin Endocrinol Metab. 2023. PMID: 37580314 Free PMC article.
-
Recommendations from the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome.Fertil Steril. 2023 Oct;120(4):767-793. doi: 10.1016/j.fertnstert.2023.07.025. Epub 2023 Aug 14. Fertil Steril. 2023. PMID: 37589624
-
International evidence-based recommendations for polycystic ovary syndrome in adolescents.BMC Med. 2025 Mar 11;23(1):151. doi: 10.1186/s12916-025-03901-w. BMC Med. 2025. PMID: 40069730 Free PMC article. Review.
Cited by
-
Intrahepatic cholestasis of pregnancy after ovarian hyperstimulation syndrome with wild-type ABCB4 gene: a peculiar case and literature review.BMC Womens Health. 2023 Jun 17;23(1):316. doi: 10.1186/s12905-023-02471-4. BMC Womens Health. 2023. PMID: 37330509 Free PMC article. Review.
-
The Thyroid Hormone Axis and Female Reproduction.Int J Mol Sci. 2023 Jun 6;24(12):9815. doi: 10.3390/ijms24129815. Int J Mol Sci. 2023. PMID: 37372963 Free PMC article. Review.
-
The Burden of Non-Alcoholic Fatty Liver Disease in Adolescents with Polycystic Ovary Syndrome: A Case-Control Study.J Clin Med. 2023 Jan 10;12(2):557. doi: 10.3390/jcm12020557. J Clin Med. 2023. PMID: 36675485 Free PMC article.
-
The Correlation between Sex Hormone-Binding Globulin and Clinical Characteristics According to Anti-Müllerian Hormone in Women with Regular Menstrual Cycles: A Prospective Study.J Pers Med. 2024 Feb 29;14(3):274. doi: 10.3390/jpm14030274. J Pers Med. 2024. PMID: 38541016 Free PMC article.
-
Genetic control of typical and atypical sex development.Nat Rev Urol. 2023 Jul;20(7):434-451. doi: 10.1038/s41585-023-00754-x. Epub 2023 Apr 5. Nat Rev Urol. 2023. PMID: 37020056 Review.
References
-
- Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855. - PubMed
-
- Azziz R, Carmina E, Chen Z, et al. . Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. - PubMed
-
- Al Wattar BH, Bueno A, Martin MG, et al. . Harmonizing research outcomes for polycystic ovary syndrome (HARP), a marathon not a sprint: current challenges and future research need. Hum Reprod. 2021;36(3):523-528. - PubMed
-
- Al Wattar BH, Teede H, Garad R, et al. . Harmonising research outcomes for polycystic ovary syndrome: an international multi-stakeholder core outcome set. Hum Reprod. 2020;35(2):404-412. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

