HAG1 and SWI3A/B control of male germ line development in P. patens suggests conservation of epigenetic reproductive control across land plants
- PMID: 33839924
- PMCID: PMC8128824
- DOI: 10.1007/s00497-021-00409-0
HAG1 and SWI3A/B control of male germ line development in P. patens suggests conservation of epigenetic reproductive control across land plants
Abstract
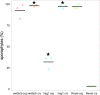
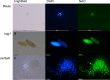
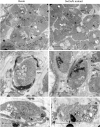
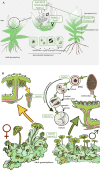
Bryophytes as models to study the male germ line: loss-of-function mutants of epigenetic regulators HAG1 and SWI3a/b demonstrate conserved function in sexual reproduction. With the water-to-land transition, land plants evolved a peculiar haplodiplontic life cycle in which both the haploid gametophyte and the diploid sporophyte are multicellular. The switch between these phases was coined alternation of generations. Several key regulators that control the bauplan of either generation are already known. Analyses of such regulators in flowering plants are difficult due to the highly reduced gametophytic generation, and the fact that loss of function of such genes often is embryo lethal in homozygous plants. Here we set out to determine gene function and conservation via studies in bryophytes. Bryophytes are sister to vascular plants and hence allow evolutionary inferences. Moreover, embryo lethal mutants can be grown and vegetatively propagated due to the dominance of the bryophyte gametophytic generation. We determined candidates by selecting single copy orthologs that are involved in transcriptional control, and of which flowering plant mutants show defects during sexual reproduction, with a focus on the under-studied male germ line. We selected two orthologs, SWI3a/b and HAG1, and analyzed loss-of-function mutants in the moss P. patens. In both mutants, due to lack of fertile spermatozoids, fertilization and hence the switch to the diploid generation do not occur. Pphag1 additionally shows arrested male and impaired female gametangia development. We analyzed HAG1 in the dioecious liverwort M. polymorpha and found that in Mphag1 the development of gametangiophores is impaired. Taken together, we find that involvement of both regulators in sexual reproduction is conserved since the earliest divergence of land plants.
Keywords: Fertilization; Gametangia; Germ line; Marchantia; Physcomitrium; Spermatozoid.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures









Similar articles
-
Transcriptomic evidence for the evolution of shoot meristem function in sporophyte-dominant land plants through concerted selection of ancestral gametophytic and sporophytic genetic programs.Mol Biol Evol. 2015 Feb;32(2):355-67. doi: 10.1093/molbev/msu303. Epub 2014 Nov 4. Mol Biol Evol. 2015. PMID: 25371433
-
MIKC* MADS-box proteins: conserved regulators of the gametophytic generation of land plants.Mol Biol Evol. 2010 May;27(5):1201-11. doi: 10.1093/molbev/msq005. Epub 2010 Jan 15. Mol Biol Evol. 2010. PMID: 20080864
-
How was apical growth regulated in the ancestral land plant? Insights from the development of non-seed plants.Plant Physiol. 2022 Aug 29;190(1):100-112. doi: 10.1093/plphys/kiac313. Plant Physiol. 2022. PMID: 35771646 Free PMC article. Review.
-
A bHLH heterodimer regulates germ cell differentiation in land plant gametophytes.Curr Biol. 2023 Nov 20;33(22):4980-4987.e6. doi: 10.1016/j.cub.2023.09.020. Epub 2023 Sep 29. Curr Biol. 2023. PMID: 37776860
-
The bryophytes Physcomitrium patens and Marchantia polymorpha as model systems for studying evolutionary cell and developmental biology in plants.Plant Cell. 2022 Jan 20;34(1):228-246. doi: 10.1093/plcell/koab218. Plant Cell. 2022. PMID: 34459922 Free PMC article. Review.
Cited by
-
Flowering plant embryos: How did we end up here?Plant Reprod. 2021 Dec;34(4):365-371. doi: 10.1007/s00497-021-00427-y. Epub 2021 Jul 27. Plant Reprod. 2021. PMID: 34313838 Free PMC article.
-
The Role of ESS2/DGCR14: Is It an Essential Factor in Splicing and Transcription?Int J Mol Sci. 2025 Apr 25;26(9):4056. doi: 10.3390/ijms26094056. Int J Mol Sci. 2025. PMID: 40362295 Free PMC article. Review.
-
Identifying Genes Associated with Female Flower Development of Phellodendron amurense Rupr. Using a Transcriptomics Approach.Genes (Basel). 2023 Mar 6;14(3):661. doi: 10.3390/genes14030661. Genes (Basel). 2023. PMID: 36980934 Free PMC article.
-
Regulation of gametangia and gametangiophore initiation in the liverwort Marchantia polymorpha.Plant Reprod. 2021 Dec;34(4):297-306. doi: 10.1007/s00497-021-00419-y. Epub 2021 Jun 11. Plant Reprod. 2021. PMID: 34117568 Review.
-
Epigenetic Dynamics and Regulation of Plant Male Reproduction.Int J Mol Sci. 2022 Sep 9;23(18):10420. doi: 10.3390/ijms231810420. Int J Mol Sci. 2022. PMID: 36142333 Free PMC article. Review.
References
-
- Aasland R, Stewart AF, Gibson T. The SANT domain: a putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem Sci. 1996;21(3):87–88. - PubMed
-
- Althoff F, Kopischke S, Zobell O, Ide K, Ishizaki K, Kohchi T, Zachgo S. Comparison of the MpEF1alpha and CaMV35 promoters for application in Marchantia polymorpha overexpression studies. Transgenic Res. 2014;23(2):235–244. - PubMed
-
- Aquea F, Timmermann T, Herrera-Vasquez A. Chemical inhibition of the histone acetyltransferase activity in Arabidopsis thaliana. Biochem Biophys Res Commun. 2017;483(1):664–668. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

