A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer
- PMID: 33840558
- PMCID: PMC8206006
- DOI: 10.1016/j.eururo.2021.03.004
A Systematic Review and Meta-analysis of the Effectiveness and Toxicities of Lutetium-177-labeled Prostate-specific Membrane Antigen-targeted Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer
Abstract
Context: Castration-resistant prostate cancer (CRPC) treatment is an evolving challenge. Prostate-specific membrane antigen (PSMA)-targeted endoradiotherapy/radioligand therapy (PRLT) with small-molecule, urea-based agents labeled with the β-particle-emitting radionuclide lutetium-177 (177Lu) is a promising new approach.
Objective: In this systematic review and meta-analysis, we evaluated the efficacy and toxicity of PRLT.
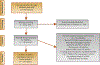
Evidence acquisition: A systematic search was performed in PubMed/Medline (last updated February 18, 2019). A total of 250 studies were reviewed, and 24 studies with 1192 patients were included in the analysis. Proportions of patients with ≥50% serum prostate-specific antigen (PSA) decrease, any PSA decrease, and any PSA increase were extracted. Proportions of patients showing any grade toxicity and those with grade 3/4 toxicities based on Common Terminology Criteria for Adverse Events (CTCAE) grading were extracted from manuscripts. Overall survival and progression-free survival were evaluated. A meta-analysis of single proportions was carried out. Furthermore, we compared the two most common PRLT agents, 177Lu-PSMA with 177Lu-PSMA-I&T, for effectiveness and toxicity.
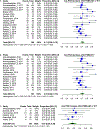
Evidence synthesis: Among the 24 included studies, 20 included data on 177Lu-PSMA-617, three included data on 177Lu-PSMA-I&T, and one study had aggregated data for 177Lu-PSMA-617 and 177Lu-PSMA-I&T. The estimated proportion of 177Lu-PSMA-617-treated patients who showed a serum PSA decrease of ≥50% with at least an 8-wk interval between therapy and PSA measurement was 0.44 (0.39; 0.50). Therapy with 177Lu-PSMA-I&T demonstrated an estimated proportion of patients with ≥50% PSA reduction to be 0.36 (0.26; 0.47). The aggregate results for men treated with more than one cycle of any kind of PRLT showed an estimated proportion of 0.46 (0.41; 0.51) for PSA response ≥50%. Regarding aggregate data from all of the PRLT agents, we found that grade 3 and 4 toxicities were uncommon, with estimated proportions from 0.01 (0.00;0.04) for nausea, fatigue, diarrhea, and elevated aspartate transaminase up to 0.08 (0.05; 0.12) for anemia. There was considerable heterogeneity among the studies in the "any-grade toxicity" groups. Meta-regression showed that more than one cycle of PRLT is associated with a greater proportion of patients with ≥50% PSA reduction. Overall survival according to pooled hazard ratios (HRs) for any PSA decline was 0.29 (0.18; 0.46), and for >50% PSA reduction was 0.67 (0.43; 1.07). Progression-free survival according to a pooled HR of >50% PSA reduction was 0.53 (0.32; 0.86).
Conclusions: The relatively high number of PSA responders alongside the low rate of severe toxicity reflects the potentially promising role of PRLT in treating CRPC. The ultimate utility of this treatment modality will become clearer as multiple prospective studies continue to accrue. In the interim, this systematic review and meta-analysis can serve as a compendium of effectiveness and adverse events associated with PRLT for treating clinicians.
Patient summary: Prostate-specific membrane antigen-targeted endoradiotherapy/radioligand therapy (PRLT) is associated with ≥50% reduction in prostate-specific antigen level in a large number of patients and a low rate of toxicity, reflecting its potential in treating castration-resistant prostate cancer. This systematic review and meta-analysis presents as a compendium of the effectiveness and adverse events related to PRLT for treating clinicians.
Keywords: Endoradiotherapy; Prostate cancer; Prostate-specific membrane antigen–targeted endoradiotherapy/radioligand therapy.
Copyright © 2021. Published by Elsevier B.V.
Figures

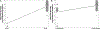
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

