Lipid hydroperoxides in nutrition, health, and diseases
- PMID: 33840675
- PMCID: PMC8062262
- DOI: 10.2183/pjab.97.010
Lipid hydroperoxides in nutrition, health, and diseases
Abstract
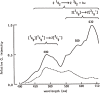
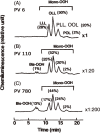
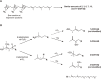
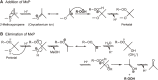
Research on lipid peroxidation in food degradation, oil and fat nutrition, and age-related diseases has gained significant international attention for the view of improvement of societal health and longevity. In order to promote basic studies on these topics, a chemiluminescence detection-high performance liquid chromatography instrument using a high-sensitivity single photon counter as a detector was developed. This instrument enabled us to selectively detect and quantify lipid hydroperoxides, a primary product of lipid peroxidation reactions, as hydroperoxide groups at the lipid class level. Furthermore, an analytical method using liquid chromatography-tandem mass spectrometry has been established to discriminate the position and stereoisomerization of hydroperoxide groups in lipid hydroperoxides. Using these two methods, the reaction mechanisms of lipid peroxidation in food and in the body have been confirmed.
Keywords: Alzheimer’s disease; atherosclerosis; chemiluminescence; immunocompetent cells; lipid hydroperoxides; rancid cooking oil.
Figures















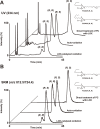

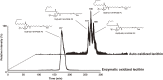
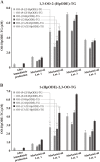
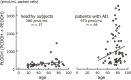


Similar articles
-
Autoxidation of methyl linoleate: identification of the bis-allylic 11-hydroperoxide.Lipids. 2000 Sep;35(9):947-52. doi: 10.1007/s11745-000-0604-0. Lipids. 2000. PMID: 11026614
-
Determination of lipid hydroperoxides in native low-density lipoprotein by a chemiluminescent flow-injection assay.Biochim Biophys Acta. 1993 Jan 10;1165(3):279-87. doi: 10.1016/0005-2760(93)90137-x. Biochim Biophys Acta. 1993. PMID: 8418885
-
Radiation induced peroxidation of polyunsaturated fatty acids: recent results on formation of hydroperoxides.Can J Physiol Pharmacol. 2001 Feb;79(2):176-9. Can J Physiol Pharmacol. 2001. PMID: 11233566
-
Lipid peroxidation: mechanisms, inhibition, and biological effects.Biochem Biophys Res Commun. 2005 Dec 9;338(1):668-76. doi: 10.1016/j.bbrc.2005.08.072. Epub 2005 Aug 19. Biochem Biophys Res Commun. 2005. PMID: 16126168 Review.
-
Immunochemical detection of lipid hydroperoxide- and aldehyde-modified proteins in diseases.Subcell Biochem. 2014;77:115-25. doi: 10.1007/978-94-007-7920-4_10. Subcell Biochem. 2014. PMID: 24374923 Review.
Cited by
-
Oxidative Stress and Lipid Dysregulation in Lipid Droplets: A Connection to Chronic Kidney Disease Revealed in Human Kidney Cells.Antioxidants (Basel). 2022 Jul 18;11(7):1387. doi: 10.3390/antiox11071387. Antioxidants (Basel). 2022. PMID: 35883878 Free PMC article.
-
Stabilization of Sunflower Oil with Biologically Active Compounds from Berries.Molecules. 2023 Apr 20;28(8):3596. doi: 10.3390/molecules28083596. Molecules. 2023. PMID: 37110830 Free PMC article.
-
Increased Cerebral Serum Amyloid A2 and Parameters of Oxidation in Arylsulfatase B (N-Acetylgalactosamine-4-Sulfatase)-Null Mice.J Alzheimers Dis Rep. 2023 Jun 2;7(1):527-534. doi: 10.3233/ADR-230028. eCollection 2023. J Alzheimers Dis Rep. 2023. PMID: 37313486 Free PMC article.
-
Inhibition of Triacylglycerol Accumulation and Oxidized Hydroperoxides in Hepatocytes by Allium cepa (Bulb).Antioxidants (Basel). 2025 May 29;14(6):653. doi: 10.3390/antiox14060653. Antioxidants (Basel). 2025. PMID: 40563288 Free PMC article.
-
Effects of Dietary Food Components on Cognitive Functions in Older Adults.Nutrients. 2021 Aug 16;13(8):2804. doi: 10.3390/nu13082804. Nutrients. 2021. PMID: 34444965 Free PMC article. Review.
References
-
- Kaneda T., Ishii S. (1954) Nutritive value or toxicity of highly unsaturated fatty acids. I. J. Biochem. 41, 327–335.
-
- Kaneda T., Sakai H., Ishii S. (1954) Nutritive value of highly unsaturated fatty acids and the origin of toxicity of fish oil. J. Jpn. Soc. Food Nutr. 7, 1–10.
-
- Kaunitz H., Slanetz C.A., Johnson R.E. (1955) Antagonism of fresh fat to the toxicity of heated and aerated cottonseed oil. J. Nutr. 55, 577–587. - PubMed
-
- Holman R.T., Greenberg S.I. (1958) A note on the toxicities of methyl oleate peroxide and ethyl linoleate peroxide. J. Am. Oil Chem. Soc. 35, 707.
-
- Kaneda T., Ishii S. (1953) Studies on the nutritive values of lipids. VII. Nutritive value or toxicity of highly unsaturated fatty acids. I. Bull. Jpn. Soc. Sci. Fish. 19, 171–177.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

