Microfluidics for the study of mechanotransduction
- PMID: 33840837
- PMCID: PMC8034607
- DOI: 10.1088/1361-6463/ab78d4
Microfluidics for the study of mechanotransduction
Abstract
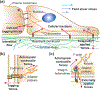
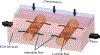
Mechanical forces regulate a diverse set of biological processes at cellular, tissue, and organismal length scales. Investigating the cellular and molecular mechanisms that underlie the conversion of mechanical forces to biological responses is challenged by limitations of traditional animal models and in vitro cell culture, including poor control over applied force and highly artificial cell culture environments. Recent advances in fabrication methods and material processing have enabled the development of microfluidic platforms that provide precise control over the mechanical microenvironment of cultured cells. These devices and systems have proven to be powerful for uncovering and defining mechanisms of mechanotransduction. In this review, we first give an overview of the main mechanotransduction pathways that function at sites of cell adhesion, many of which have been investigated with microfluidics. We then discuss how distinct microfluidic fabrication methods can be harnessed to gain biological insight, with description of both monolithic and replica molding approaches. Finally, we present examples of how microfluidics can be used to apply both solid forces (substrate mechanics, strain, and compression) and fluid forces (luminal, interstitial) to cells. Throughout the review, we emphasize the advantages and disadvantages of different fabrication methods and applications of force in order to provide perspective to investigators looking to apply forces to cells in their own research.
Figures


References
-
- Geiger B, Spatz JP and Bershadsky AD 2009. Environmental sensing through focal adhesions Nat. Rev. Mol. Cell Biol 10 21–33 - PubMed
-
- Charras G and Yap AS 2018. Tensile Forces and Mechanotransduction at Cell–Cell Junctions Curr. Biol 28 R445–57 - PubMed
-
- Ng JL, Shin Y and Chung S 2012. Microfluidic platforms for the study of cancer metastasis Biomed. Eng. Lett 2 72–7
-
- Wong KHK, Chan JM, Kamm RD and Tien J 2012. Microfluidic Models of Vascular Functions Annu. Rev. Biomed. Eng 14 205–30 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
