Anti-tumor activities of Panax quinquefolius saponins and potential biomarkers in prostate cancer
- PMID: 33841008
- PMCID: PMC8020356
- DOI: 10.1016/j.jgr.2019.12.007
Anti-tumor activities of Panax quinquefolius saponins and potential biomarkers in prostate cancer
Abstract
Background: Prostate carcinoma is the second most common cancer among men worldwide. Developing new therapeutic approaches and diagnostic biomarkers for prostate cancer (PC) is a significant need. The Chinese herbal medicine Panax quinquefolius saponins (PQS) have been reported to show anti-tumor effects. We hypothesized that PQS exhibits anti-cancer activity in human PC cells and we aimed to search for novel biomarkers allowing early diagnosis of PC.
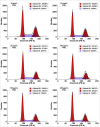
Methods: We used the human PC cell line DU145 and the prostate epithelial cell line PNT2 to perform cell viability assays, flow cytometric analysis of the cell cycle, and FACS-based apoptosis assays. Microarray-based gene expression analysis was used to display specific gene expression patterns and to search for novel biomarkers. Western blot and quantitative real-time PCR were performed to demonstrate the expression levels of multiple cancer-related genes.
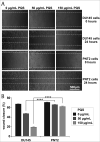
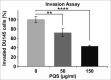
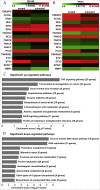
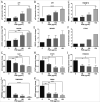
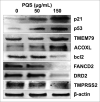
Results: Our data showed that PQS inhibited the viability of DU145 cells and induced cell cycle arrest at the G1 phase. A significant decrease in DU145 cell invasion and migration were observed after 24 h treatment by PQS. PQS up-regulated the expression levels of p21, p53, TMEM79, ACOXL, ETV5, and SPINT1 while it down-regulated the expression levels of bcl2, STAT3, FANCD2, DRD2, and TMPRSS2.
Conclusion: PQS promoted cells apoptosis and inhibited the proliferation of DU145 cells, which suggests that PQS may be effective for treating PC. TMEM79 and ACOXL were expressed significantly higher in PNT2 than in DU145 cells and could be novel biomarker candidates for PC diagnosis.
Keywords: ACOXL, Acyl-CoA oxidase-like protein; Chinese medicinal herbs; DRD2, dopamine receptor D2; ETV5, ETS variant 5; FACS, fluorescence-activated cell sorting; FANCD2, fanconi anemia group D2; PC, prostate cancer; PQS, Panax quinquefolius saponins; Panax quinquefolius; Potential biomarkers; Prostate cancer cells; SPINT1, serine peptidase inhibitor Kunitz type 1; STAT3, signal transducer and activator of transcription 3; TCM, Traditional Chinese Medicine; TMEM79, transmembrane protein 79; TMPRSS2, transmembrane protease serine 2; bcl2, B-cell lymphoma 2; p21, cyclin-dependent kinase inhibitor p21; p53, tumor suppressor p53; qRT-PCR, quantitative real-time PCR; saponins.
© 2020 The Korean Society of Ginseng. Publishing services by Elsevier B.V.
Conflict of interest statement
All authors have no conflicts of interest to declare.
Figures
Similar articles
-
Saponins (Ginsenosides) from the Leaves of Panax quinquefolius Ameliorated Acetaminophen-Induced Hepatotoxicity in Mice.J Agric Food Chem. 2017 May 10;65(18):3684-3692. doi: 10.1021/acs.jafc.7b00610. Epub 2017 Apr 25. J Agric Food Chem. 2017. PMID: 28429935
-
Nephroprotective Effects of Saponins from Leaves of Panax quinquefolius against Cisplatin-Induced Acute Kidney Injury.Int J Mol Sci. 2017 Jul 13;18(7):1407. doi: 10.3390/ijms18071407. Int J Mol Sci. 2017. PMID: 28703736 Free PMC article.
-
Panax quinquefolium saponin combined with dual antiplatelet drugs inhibits platelet adhesion to injured HUVECs via PI3K/AKT and COX pathways.J Ethnopharmacol. 2016 Nov 4;192:10-19. doi: 10.1016/j.jep.2016.07.015. Epub 2016 Jul 8. J Ethnopharmacol. 2016. PMID: 27401285
-
Panax quinquefolius saponin inhibits vascular smooth muscle cell calcification via activation of nuclear factor-erythroid 2-related factor 2.BMC Complement Med Ther. 2023 Apr 21;23(1):129. doi: 10.1186/s12906-023-03961-6. BMC Complement Med Ther. 2023. PMID: 37085826 Free PMC article.
-
Gene methylation in gastric cancer.Clin Chim Acta. 2013 Sep 23;424:53-65. doi: 10.1016/j.cca.2013.05.002. Epub 2013 May 10. Clin Chim Acta. 2013. PMID: 23669186 Review.
Cited by
-
Mechanism allowing biochar to aid in arbuscular mycorrhizal colonization in Panax quinquefolius L. roots and improve secondary metabolite production.Mycorrhiza. 2025 Mar 19;35(2):23. doi: 10.1007/s00572-025-01195-7. Mycorrhiza. 2025. PMID: 40106050
-
Anticancer Potential of Raddeanin A, a Natural Triterpenoid Isolated from Anemone raddeana Regel.Molecules. 2020 Feb 25;25(5):1035. doi: 10.3390/molecules25051035. Molecules. 2020. PMID: 32106609 Free PMC article. Review.
-
Region-Specific Biomarkers and Their Mechanisms in the Treatment of Lung Adenocarcinoma: A Study of Panax quinquefolius from Wendeng, China.Molecules. 2021 Nov 12;26(22):6829. doi: 10.3390/molecules26226829. Molecules. 2021. PMID: 34833921 Free PMC article.
-
Effect of ginseng and ginsenosides on attention deficit hyperactivity disorder: A systematic review.J Ginseng Res. 2024 Sep;48(5):437-448. doi: 10.1016/j.jgr.2024.05.006. Epub 2024 May 28. J Ginseng Res. 2024. PMID: 39263306 Free PMC article. Review.
-
KRG and its major ginsenosides do not show distinct steroidogenic activities examined by the OECD test guideline 440 and 456 assays.J Ginseng Res. 2023 May;47(3):385-389. doi: 10.1016/j.jgr.2022.09.002. Epub 2022 Oct 5. J Ginseng Res. 2023. PMID: 37252278 Free PMC article.
References
-
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67:7–30. 2017. - PubMed
-
- Sadeghi-Gandomani H.R., Yousefi M., Rahimi S., Yousefi S., Karimi-Rozveh A., Hosseini S., Mahabadi A.A., Abarqui H.F., Borujeni N.N., Salehiniya H. The incidence, risk factors, and knowledge about the prostate cancer through worldwide and Iran. WCRJ. 2017;4(4):e972. 2017.
-
- Mottet N., Bellmunt J., Briers E., Bolla M., Cornford P., De Santis M., Henry A., Joniau S., Lam T., Mason M.D. European Association of Urology; 2016. EAU - ESTRO - SIOG guidelines on prostate cancer. 2016. - PubMed
-
- Pin E., Henjes F., Hong M.G., Wiklund F., Magnusson P., Bjartell A. Identification of a novel autoimmune peptide epitope of prostein in prostate cancer. J Proteome Res. 2017;16:204–216. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

