Measuring fidelity to delivery of a new smoking cessation intervention integrated into routine tuberculosis care in Pakistan and Bangladesh: Contextual differences and opportunities
- PMID: 33841063
- PMCID: PMC8029647
- DOI: 10.18332/tid/133054
Measuring fidelity to delivery of a new smoking cessation intervention integrated into routine tuberculosis care in Pakistan and Bangladesh: Contextual differences and opportunities
Abstract
Introduction: Tobacco smoking among tuberculosis (TB) patients leads to poorer treatment outcomes. Smoking cessation support should be integrated into routine TB care. We measured healthcare providers' fidelity to a smoking cessation intervention integrated into routine TB care, in Bangladesh and Pakistan. We aimed to understand the role of providers and settings in the implementation of behavior support (BS) messages for TB and smoking cessation.
Methods: The integrated BS intervention was implemented in TB clinics (24 public and 1 private). Cross-sectional data were collected on the fidelity of delivery of the BS intervention using a predefined fidelity index based on an existing validated method of measuring intervention fidelity. Audio-recordings of patient-provider BS sessions were coded using the fidelity index. Intervention fidelity was presented as the proportion of sessions that implemented BS messages.
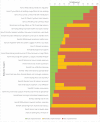
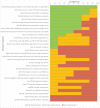
Results: A total of 96 sessions were conducted, 37 in Bangladesh and 59 in Pakistan. In public settings, TB medication advice was offered in 91.9% (95% CI: 78.7- 97.2) of sessions in Bangladesh, and in 75.5% (95% CI: 62.4-85.1) of sessions in Pakistan; whilst it was offered in 83.3% (95% CI: 43.7-97.0) of sessions in the private setting in Pakistan. Patients' smoking status was assessed in 70.3% (95% CI: 54.2-82.5) of sessions in Bangladesh, and in 34.0% (95% CI: 22.7-47.4) of sessions in the public setting and in 66.7% (95% CI: 30.0-90.3) of sessions in the private setting in Pakistan. A quit date was set in 32.4% (95% CI: 19.6-48.5) of all sessions in Bangladesh, and in 33.3% (95% CI: 9.6-70.0) of all sessions in the public setting in Pakistan.
Conclusions: Fidelity to the intended delivery of the intervention was found to be high for TB-related messages but not for smoking cessation messages. Clinic contexts may play a mediating role in health workers' opportunities to deliver the intervention as planned.
Trial registration: International Standard Randomized Clinical Trial Number (ISRCTN43811467). Registered 23 March 2016, https://doi.org/10.1186/ISRCTN43811467.
Keywords: South Asia; behavior change; primary health care; tobacco.
© 2021 Boeckmann M. et al.
Conflict of interest statement
The authors have each completed and submitted an ICMJE form for disclosure of potential conflicts of interest. The authors declare that they have no competing interests, financial or otherwise, related to the current work. K. Siddiqi reports grants from European Commission H2020, and non-financial support from Aflofarm, during the conduct of the study. D. Kotz reports that he received an unrestricted grant from Pfizer in 2009 for an investigator-initiated trial on the effectiveness of practice nurse counselling and varenicline for smoking cessation in primary care (Dutch Trial Register NTR3067; DOI: 10.1111/add.13927).
Figures
References
-
- World Health Organization . A WHO/The Union Monograph on TB and Tobacco Control. Geneva: World Health Organization; 2007. https://apps.who.int/iris/bitstream/handle/10665/43812/9789241596220_eng.... Accessed May 30, 2017.
LinkOut - more resources
Full Text Sources
Other Literature Sources