Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration
- PMID: 33841102
- PMCID: PMC8032904
- DOI: 10.3389/fncel.2021.652593
Phosphoinositides: Roles in the Development of Microglial-Mediated Neuroinflammation and Neurodegeneration
Abstract
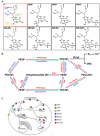
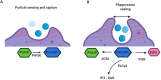
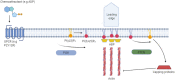
Microglia are increasingly recognized as vital players in the pathology of a variety of neurodegenerative conditions including Alzheimer's (AD) and Parkinson's (PD) disease. While microglia have a protective role in the brain, their dysfunction can lead to neuroinflammation and contributes to disease progression. Also, a growing body of literature highlights the seven phosphoinositides, or PIPs, as key players in the regulation of microglial-mediated neuroinflammation. These small signaling lipids are phosphorylated derivates of phosphatidylinositol, are enriched in the brain, and have well-established roles in both homeostasis and disease.Disrupted PIP levels and signaling has been detected in a variety of dementias. Moreover, many known AD disease modifiers identified via genetic studies are expressed in microglia and are involved in phospholipid metabolism. One of these, the enzyme PLCγ2 that hydrolyzes the PIP species PI(4,5)P2, displays altered expression in AD and PD and is currently being investigated as a potential therapeutic target.Perhaps unsurprisingly, neurodegenerative conditions exhibiting PIP dyshomeostasis also tend to show alterations in aspects of microglial function regulated by these lipids. In particular, phosphoinositides regulate the activities of proteins and enzymes required for endocytosis, toll-like receptor signaling, purinergic signaling, chemotaxis, and migration, all of which are affected in a variety of neurodegenerative conditions. These functions are crucial to allow microglia to adequately survey the brain and respond appropriately to invading pathogens and other abnormalities, including misfolded proteins. AD and PD therapies are being developed to target many of the above pathways, and although not yet investigated, simultaneous PIP manipulation might enhance the beneficial effects observed. Currently, only limited therapeutics are available for dementia, and although these show some benefits for symptom severity and progression, they are far from curative. Given the importance of microglia and PIPs in dementia development, this review summarizes current research and asks whether we can exploit this information to design more targeted, or perhaps combined, dementia therapeutics. More work is needed to fully characterize the pathways discussed in this review, but given the strength of the current literature, insights in this area could be invaluable for the future of neurodegenerative disease research.
Keywords: Alzheimer’s disease; Parkinson’s disease; chemotaxis; microglia; neurodegeneration; neuroinflammation; phagocytosis; phosphoinositols.
Copyright © 2021 Phillips and Maguire.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Microglial ion channels: Key players in non-cell autonomous neurodegeneration.Neurobiol Dis. 2022 Nov;174:105861. doi: 10.1016/j.nbd.2022.105861. Epub 2022 Sep 14. Neurobiol Dis. 2022. PMID: 36115552 Free PMC article. Review.
-
Emerging roles of microglial cathepsins in neurodegenerative disease.Brain Res Bull. 2018 May;139:144-156. doi: 10.1016/j.brainresbull.2018.02.014. Epub 2018 Feb 15. Brain Res Bull. 2018. PMID: 29454581 Review.
-
Targeting microglial autophagic degradation in NLRP3 inflammasome-mediated neurodegenerative diseases.Ageing Res Rev. 2021 Jan;65:101202. doi: 10.1016/j.arr.2020.101202. Epub 2020 Nov 5. Ageing Res Rev. 2021. PMID: 33161129 Review.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Microglia in Parkinson's Disease.Adv Exp Med Biol. 2019;1175:335-353. doi: 10.1007/978-981-13-9913-8_13. Adv Exp Med Biol. 2019. PMID: 31583594 Review.
Cited by
-
Differential Effects of Post-translational Modifications on the Membrane Interaction of Huntingtin Protein.ACS Chem Neurosci. 2024 Jun 19;15(12):2408-2419. doi: 10.1021/acschemneuro.4c00091. Epub 2024 May 16. ACS Chem Neurosci. 2024. PMID: 38752226 Free PMC article.
-
Machine learning model base on metabolomics and proteomics to predict cognitive impairment in Parkinson's disease.NPJ Parkinsons Dis. 2024 Oct 11;10(1):187. doi: 10.1038/s41531-024-00795-y. NPJ Parkinsons Dis. 2024. PMID: 39394257 Free PMC article.
-
Neuroinflammation Markers in Tear Fluid of Mild Alzheimer's Disease.J Mol Neurosci. 2025 Jun 5;75(2):73. doi: 10.1007/s12031-025-02368-x. J Mol Neurosci. 2025. PMID: 40471493 Free PMC article.
-
The effects and potential of microglial polarization and crosstalk with other cells of the central nervous system in the treatment of Alzheimer's disease.Neural Regen Res. 2023 May;18(5):947-954. doi: 10.4103/1673-5374.355747. Neural Regen Res. 2023. PMID: 36254973 Free PMC article. Review.
-
TREM2 Modulates Postoperative Cognitive Function in Aged Mice by Inhibiting the NLRP3/caspase-1 Pathway and Apoptosis via PLCγ2 Activation.Mol Neurobiol. 2025 Jul;62(7):8986-8999. doi: 10.1007/s12035-025-04820-w. Epub 2025 Mar 12. Mol Neurobiol. 2025. PMID: 40075039
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

