The Effect of GLT-1 Upregulation on Extracellular Glutamate Dynamics
- PMID: 33841104
- PMCID: PMC8032948
- DOI: 10.3389/fncel.2021.661412
The Effect of GLT-1 Upregulation on Extracellular Glutamate Dynamics
Abstract
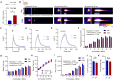
Pharmacological upregulation of glutamate transporter-1 (GLT-1), commonly achieved using the beta-lactam antibiotic ceftriaxone, represents a promising therapeutic strategy to accelerate glutamate uptake and prevent excitotoxic damage in neurological conditions. While excitotoxicity is indeed implicated in numerous brain diseases, it is typically restricted to select vulnerable brain regions, particularly in early disease stages. In healthy brain tissue, the speed of glutamate uptake is not constant and rather varies in both an activity- and region-dependent manner. Despite the widespread use of ceftriaxone in disease models, very little is known about how such treatments impact functional measures of glutamate uptake in healthy tissue, and whether GLT-1 upregulation can mask the naturally occurring activity-dependent and regional heterogeneities in uptake. Here, we used two different compounds, ceftriaxone and LDN/OSU-0212320 (LDN), to upregulate GLT-1 in healthy wild-type mice. We then used real-time imaging of the glutamate biosensor iGluSnFR to investigate functional consequences of GLT-1 upregulation on activity- and regional-dependent variations in glutamate uptake capacity. We found that while both ceftriaxone and LDN increased GLT-1 expression in multiple brain regions, they did not prevent activity-dependent slowing of glutamate clearance nor did they speed basal clearance rates, even in areas characterized by slow uptake (e.g., striatum). Unexpectedly, ceftriaxone but not LDN decreased glutamate release in the cortex, suggesting that ceftriaxone may alter release properties independent of its effects on GLT-1 expression. In sum, our data demonstrate the complexities of glutamate uptake by showing that GLT-1 expression does not necessarily translate to accelerated uptake. Furthermore, these data suggest that the mechanisms underlying activity- and regional-dependent differences in glutamate dynamics are independent of GLT-1 expression levels.
Keywords: ceftriaxone; glutamate transporter; iGluSnFR; neurotransmission; uptake.
Copyright © 2021 Wilkie, Barron, Brymer, Barnes, Nafar and Parsons.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



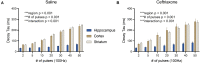


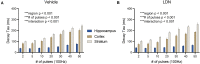
Similar articles
-
Hippocampal Synaptic Dysfunction in a Mouse Model of Huntington Disease Is Not Alleviated by Ceftriaxone Treatment.eNeuro. 2020 May 21;7(3):ENEURO.0440-19.2020. doi: 10.1523/ENEURO.0440-19.2020. Print 2020 May/Jun. eNeuro. 2020. PMID: 32354757 Free PMC article.
-
Region- and Activity-Dependent Regulation of Extracellular Glutamate.J Neurosci. 2018 Jun 6;38(23):5351-5366. doi: 10.1523/JNEUROSCI.3213-17.2018. Epub 2018 May 14. J Neurosci. 2018. PMID: 29760178 Free PMC article.
-
Contrasting the Role of xCT and GLT-1 Upregulation in the Ability of Ceftriaxone to Attenuate the Cue-Induced Reinstatement of Cocaine Seeking and Normalize AMPA Receptor Subunit Expression.J Neurosci. 2017 Jun 14;37(24):5809-5821. doi: 10.1523/JNEUROSCI.3717-16.2017. Epub 2017 May 11. J Neurosci. 2017. PMID: 28495973 Free PMC article.
-
Molecular changes evoked by the beta-lactam antibiotic ceftriaxone across rodent models of substance use disorder and neurological disease.Neurosci Biobehav Rev. 2020 Aug;115:116-130. doi: 10.1016/j.neubiorev.2020.05.016. Epub 2020 May 30. Neurosci Biobehav Rev. 2020. PMID: 32485268 Free PMC article. Review.
-
Current approaches to enhance glutamate transporter function and expression.J Neurochem. 2015 Sep;134(6):982-1007. doi: 10.1111/jnc.13200. Epub 2015 Aug 14. J Neurochem. 2015. PMID: 26096891 Review.
Cited by
-
Glial Glutamate Transporter Modulation Prevents Development of Complete Freund's Adjuvant-Induced Hyperalgesia and Allodynia in Mice.Brain Sci. 2023 May 16;13(5):807. doi: 10.3390/brainsci13050807. Brain Sci. 2023. PMID: 37239279 Free PMC article.
-
Blueprint of Collapse: Precision Biomarkers, Molecular Cascades, and the Engineered Decline of Fast-Progressing ALS.Int J Mol Sci. 2025 Aug 21;26(16):8072. doi: 10.3390/ijms26168072. Int J Mol Sci. 2025. PMID: 40869392 Free PMC article. Review.
-
Bioactive human Alzheimer brain soluble Aβ: pathophysiology and therapeutic opportunities.Mol Psychiatry. 2022 Aug;27(8):3182-3191. doi: 10.1038/s41380-022-01589-5. Epub 2022 Apr 28. Mol Psychiatry. 2022. PMID: 35484241 Review.
-
Current state of neuroprotective therapy using antibiotics in human traumatic brain injury and animal models.BMC Neurosci. 2024 Feb 29;25(1):10. doi: 10.1186/s12868-024-00851-6. BMC Neurosci. 2024. PMID: 38424488 Free PMC article. Review.
-
Glutamate indicators with increased sensitivity and tailored deactivation rates.bioRxiv [Preprint]. 2025 Mar 31:2025.03.20.643984. doi: 10.1101/2025.03.20.643984. bioRxiv. 2025. PMID: 40196590 Free PMC article. Preprint.
References
-
- Agostini J. F., Costa N. L. F., Bernardo H. T., Baldin S. L., Mendes N. V., de Pieri Pickler K., et al. (2020). Ceftriaxone attenuated anxiety-like behavior and enhanced brain glutamate transport in zebrafish subjected to alcohol withdrawal. Neurochem. Res. 45 1526–1535. 10.1007/s11064-020-03008-z - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

