Fatty Acid-Binding Protein 3 Expression in the Brain and Skin in Human Synucleinopathies
- PMID: 33841128
- PMCID: PMC8026871
- DOI: 10.3389/fnagi.2021.648982
Fatty Acid-Binding Protein 3 Expression in the Brain and Skin in Human Synucleinopathies
Abstract
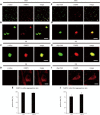
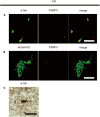
Parkinson's disease (PD) and multiple system atrophy are types of adult-onset neurodegenerative disorders named synucleinopathies, which are characterized by prominent intracellular α-synuclein (αSyn) aggregates. We have previously found that αSyn aggregates and the vulnerability of dopaminergic neurons in the mouse brain are partly associated with the expression of fatty acid-binding protein 3 (FABP3, heart FABP). However, it remains to be elucidated whether FABP3 accumulation is associated with αSyn aggregates in human tissues. Here, we histologically studied FABP3 expression in human tissues obtained from patients with synucleinopathies, patients with Alzheimer disease (AD) and controls. We found that (1) a variety of neurons expressed the FABP3 protein in human brain tissues, (2) FABP3 was colocalized with αSyn aggregates in the brains of individuals with synucleinopathies but not with amyloid β or p-tau aggregates in the brains of individuals with AD, and (3) FABP3 was not present in p-αSyn deposits in biopsied skin tissues from individuals with PD. These findings suggest that FABP3 expression is associated with αSyn aggregation in synucleinopathies and provide new insights into the involvement of FABP3 in synucleinopathies.
Keywords: Parkinson’s disease; fatty acid-binding protein; human; multiple system atrophy; α-synuclein.
Copyright © 2021 Oizumi, Yamasaki, Suzuki, Hasegawa, Sugimura, Baba, Fukunaga and Takeda.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Suppression of α-synuclein propagation after intrastriatal injection in FABP3 null mice.Brain Res. 2021 Jun 1;1760:147383. doi: 10.1016/j.brainres.2021.147383. Epub 2021 Feb 24. Brain Res. 2021. PMID: 33636166
-
Development of FABP3 ligands that inhibit arachidonic acid-induced α-synuclein oligomerization.Brain Res. 2019 Mar 15;1707:190-197. doi: 10.1016/j.brainres.2018.11.036. Epub 2018 Nov 26. Brain Res. 2019. PMID: 30496735
-
An α-synuclein decoy peptide prevents cytotoxic α-synuclein aggregation caused by fatty acid binding protein 3.J Biol Chem. 2021 Jan-Jun;296:100663. doi: 10.1016/j.jbc.2021.100663. Epub 2021 Apr 20. J Biol Chem. 2021. PMID: 33862084 Free PMC article.
-
Impact of fatty acid-binding proteins and dopamine receptors on α-synucleinopathy.J Pharmacol Sci. 2022 Feb;148(2):248-254. doi: 10.1016/j.jphs.2021.12.003. Epub 2021 Dec 14. J Pharmacol Sci. 2022. PMID: 35063140 Review.
-
Lipids as Trans-Acting Effectors for α-Synuclein in the Pathogenesis of Parkinson's Disease.Front Neurosci. 2019 Jul 3;13:693. doi: 10.3389/fnins.2019.00693. eCollection 2019. Front Neurosci. 2019. PMID: 31333408 Free PMC article. Review.
Cited by
-
Exploring the Role of Lipid-Binding Proteins and Oxidative Stress in Neurodegenerative Disorders: A Focus on the Neuroprotective Effects of Nutraceutical Supplementation and Physical Exercise.Antioxidants (Basel). 2022 Oct 27;11(11):2116. doi: 10.3390/antiox11112116. Antioxidants (Basel). 2022. PMID: 36358488 Free PMC article. Review.
-
Fatty Acid-Binding Proteins: Their Roles in Ischemic Stroke and Potential as Drug Targets.Int J Mol Sci. 2022 Aug 25;23(17):9648. doi: 10.3390/ijms23179648. Int J Mol Sci. 2022. PMID: 36077044 Free PMC article. Review.
-
Preliminary evaluation of the proteomic profiling in the hippocampus of aged grazing cattle.Front Aging Neurosci. 2023 Oct 27;15:1274073. doi: 10.3389/fnagi.2023.1274073. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37965495 Free PMC article.
-
Synthesis and Bioactivity Evaluation of a Novel 1,2,4-Oxadiazole Derivative in vitro and in 3×Tg Mice.Drug Des Devel Ther. 2022 Sep 25;16:3285-3296. doi: 10.2147/DDDT.S372750. eCollection 2022. Drug Des Devel Ther. 2022. PMID: 36187086 Free PMC article.
-
Radiosynthesis, structural identification and in vitro tissue binding study of [18F]FNA-S-ACooP, a novel radiopeptide for targeted PET imaging of fatty acid binding protein 3.EJNMMI Radiopharm Chem. 2024 Feb 23;9(1):16. doi: 10.1186/s41181-024-00245-3. EJNMMI Radiopharm Chem. 2024. PMID: 38393497 Free PMC article.
References
-
- Chiasserini D., Biscetti L., Eusebi P., Salvadori N., Frattini G., Simoni S., et al. (2017). Differential role of CSF fatty acid binding protein 3, alpha-synuclein, and Alzheimer’s disease core biomarkers in Lewy body disorders and Alzheimer’s dementia. Alzheimers Res. Ther. 9:52. 10.1186/s13195-017-0276-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

