Antiretroviral Long-Term Efficacy and Resistance of Lopinavir/Ritonavir Plus Lamivudine in HIV-1-Infected Treatment-Naïve Patients (ALTERLL): 144-Week Results of a Randomized, Open-Label, Non-Inferiority Study From Guangdong, China
- PMID: 33841131
- PMCID: PMC8027496
- DOI: 10.3389/fphar.2020.569766
Antiretroviral Long-Term Efficacy and Resistance of Lopinavir/Ritonavir Plus Lamivudine in HIV-1-Infected Treatment-Naïve Patients (ALTERLL): 144-Week Results of a Randomized, Open-Label, Non-Inferiority Study From Guangdong, China
Abstract
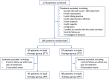
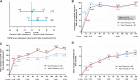
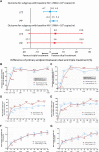
Dual therapy with lopinavir/ritonavir (LPV/r) plus lamivudine (3TC) has been demonstrated to be non-inferior to the triple drug regimen including LPV/r plus two nucleoside reverse transcriptase inhibitors (NRTIs) in 48-week studies. However, little is known about the long-term efficacy and drug resistance of this simplified strategy. A randomized, controlled, open-label, non-inferiority trial (ALTERLL) was conducted to assess the efficacy, drug resistance, and safety of dual therapy with LPV/r plus 3TC (DT group), compared with the first-line triple-therapy regimen containing tenofovir (TDF), 3TC plus efavirenz (EFV) (TT group) in antiretroviral therapy (ART)-naïve HIV-1-infected adults in Guangdong, China. The primary endpoint was the proportion of patients with plasma HIV-1 RNA < 50 copies/ml at week 144. Between March 1 and December 31, 2015, a total of 196 patients (from 274 patients screened) were included and randomly assigned to either the DT group (n = 99) or the TT group (n = 97). In the primary intention-to-treat (ITT) analysis at week 144, 95 patients (96%) in the DT group and 93 patients (95.9%) in the TT group achieved virological inhibition with plasma HIV-1 RNA <50 copies/ml (difference: 0.1%; 95% CI, -4.6-4.7%), meeting the criteria for non-inferiority. The DT group did not show significant differences in the mean increase in CD4+ cell count (247.0 vs. 204.5 cells/mm3; p = 0.074) or the CD4/CD8 ratio (0.47 vs. 0.49; p = 0.947) from baseline, or the inflammatory biomarker levels through week 144 compared with the TT group. For the subgroup analysis, baseline high viremia (HIV-1 RNA > 100,000 copies/ml) and genotype BC did not affect the primary endpoint or the mean increase in CD4+ cell count or CD4/CD8 ratio from baseline at week 144. However, in patients with genotype AE, the DT group showed a higher mean increase in CD4+ cell count from baseline through 144 weeks than the TT group (308.7 vs. 209.4 cells/mm3; p = 0.038). No secondary HIV resistance was observed in either group. Moreover, no severe adverse event (SAE) or death was observed in any group. Nonetheless, more patients in the TT group (6.1%) discontinued the assigned regimen than those in the DT group (1%) due to adverse events. Dual therapy with LPV/r plus 3TC manifests long-term non-inferior therapeutic efficacy, low drug resistance, good safety, and tolerability compared with the first-line triple-therapy regimen in Guangdong, China, indicating dual therapy is a viable alternative in resource-limited areas. Clinical Trial Registration: [http://www.chictr.org.cn], identifier [ChiCTR1900024611].
Keywords: antiretroviral therapy; efavirenz; inflammatory biomarker; lopinavir/ritonavir; randomized controlled study; simplified regimen.
Copyright © 2021 Guo, He, Chen, Chen, Chen, Lan, Wang, Du, Zhong, Li, Liu, Li, Hu, Tang, Cai and LI.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial.Lancet Infect Dis. 2014 Jul;14(7):572-80. doi: 10.1016/S1473-3099(14)70736-4. Epub 2014 Apr 27. Lancet Infect Dis. 2014. PMID: 24783988 Clinical Trial.
-
Dual therapy with lopinavir/ritonavir plus lamivudine could be a viable alternative for antiretroviral-therapy-naive adults with HIV-1 infection regardless of HIV viral load or subgenotype in resource-limited settings: A randomised, open-label and non-inferiority study from China.Indian J Med Microbiol. 2018 Oct-Dec;36(4):513-516. doi: 10.4103/ijmm.IJMM_18_172. Indian J Med Microbiol. 2018. PMID: 30880698 Clinical Trial.
-
Efavirenz-based simplification after successful early lopinavir-boosted-ritonavir-based therapy in HIV-infected children in Burkina Faso and Côte d'Ivoire: the MONOD ANRS 12206 non-inferiority randomised trial.BMC Med. 2017 Apr 24;15(1):85. doi: 10.1186/s12916-017-0842-4. BMC Med. 2017. PMID: 28434406 Free PMC article. Clinical Trial.
-
Assessing the Efficacy of Lopinavir/Ritonavir-Based Preferred and Alternative Second-Line Regimens in HIV-Infected Patients: A Meta-Analysis of Key Evidence to Support WHO Recommendations.Front Pharmacol. 2018 Aug 14;9:890. doi: 10.3389/fphar.2018.00890. eCollection 2018. Front Pharmacol. 2018. PMID: 30174599 Free PMC article. Review.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
Cited by
-
INSTIs-centered antiviral regimens for first-line treatment of HIV/AIDS: a network meta-analysis and cost-effectiveness analysis.BMC Infect Dis. 2025 Apr 25;25(1):604. doi: 10.1186/s12879-025-10858-x. BMC Infect Dis. 2025. PMID: 40281525 Free PMC article.
-
The impact of climate, weather, seasonal transitions, and diurnal rhythms on gut microbiota and immune homeostasis.Antonie Van Leeuwenhoek. 2025 Jun 1;118(7):86. doi: 10.1007/s10482-025-02097-6. Antonie Van Leeuwenhoek. 2025. PMID: 40450648 Review.
-
Early on-treatment plasma interleukin-18 as a promising indicator for long-term virological response in patients with HIV-1 infection.Front Med (Lausanne). 2023 Jun 13;10:1170208. doi: 10.3389/fmed.2023.1170208. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37384047 Free PMC article.
-
Metagenomic profiling of gut microbime: associating their role with the advancement of diabetic nephropathy.Antonie Van Leeuwenhoek. 2025 Aug 22;118(9):135. doi: 10.1007/s10482-025-02141-5. Antonie Van Leeuwenhoek. 2025. PMID: 40844735 Review.
-
Neuroinflammation and energy metabolism: a dual perspective on ischemic stroke.J Transl Med. 2025 Apr 10;23(1):413. doi: 10.1186/s12967-025-06440-3. J Transl Med. 2025. PMID: 40211331 Free PMC article. Review.
References
-
- Arribas J. R., Girard P. M., Landman R., Pich J., Mallolas J., Martínez-Rebollar M., et al. (2015). Dual treatment with lopinavir/ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect. Dis. 15 (7), 785–792. 10.1016/s1473-3099(15)00096-1 - DOI - PubMed
-
- Bastard J. P., Soulie C., Fellahi S., Haïm-Boukobza S., Simon A., Katlama C., et al. (2012). Circulating interleukin-6 levels correlate with residual HIV viraemia and markers of immune dysfunction in treatment-controlled HIV-infected patients. Antivir. Ther. 17 (5), 915–919. 10.3851/imp2093 - DOI - PubMed
-
- Blasco A. J., Llibre J. M., Arribas J. R., Boix V., Clotet B., Domingo P., et al. (2013). Analysis of costs and cost-effectiveness of preferred GESIDA/National AIDS Plan regimens for initial antiretroviral therapy in human immunodeficiency virus infected adult patients in 2013. Enferm. Infecc. Microbiol. Clín. 31, 568–578. 10.1016/j.eimc.2013.06.002 - DOI - PubMed
-
- Cahn P., Andrade-Villanueva J., Arribas J. R., Gatell J. M., Lama J. R., Norton M., et al. (2014). Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviraltherapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect. Dis. 14, 572–580. 10.1016/s1473-3099(14)70736-4 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

