Attitudes and Practices of Immune Checkpoint Inhibitors in Chinese Patients With Cancer: A National Cross-Sectional Survey
- PMID: 33841138
- PMCID: PMC8025873
- DOI: 10.3389/fphar.2021.583126
Attitudes and Practices of Immune Checkpoint Inhibitors in Chinese Patients With Cancer: A National Cross-Sectional Survey
Abstract
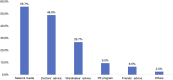
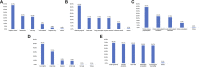
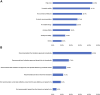
Immune-checkpoint inhibitors (ICIs) are revolutionizing the field of immuno-oncology. Side effects and tumor microenvironment currently represent the most significant obstacles to using ICIs. In this study, we conducted an extensive cross-sectional survey to investigate the concept and practices regarding the use of ICIs in cancer patients in China. The results provide real-world data on the adverse events (AEs) of ICIs and the factors influencing the use of ICIs. This survey was developed by the Expert Committee on Immuno-Oncology of the Chinese Society of Clinical Oncology (CSCO-IO) and the Expert Committee on Patient Education of the Chinese Society of Clinical Oncology (CSCO-PE). The surveys were distributed using a web-based platform between November 29, 2019 and December 21, 2019. A total of 1,575 patients were included. High costs (43.9%), uncertainty about drug efficacy (41.2%), and no reimbursement from medical insurance (32.4%) were the factors that prevented the patients from using ICIs. The patients were most concerned about the onset time or effective duration of ICIs (40.3%), followed by the indications of ICIs and pre-use evaluation (33.4%). Moreover, 9.0, 57.1, 21.0, and 12.9% of the patients reported tumor disappearance, tumor volume reduction, no change in tumor volume, and increased tumor volume. Among the patients who received ICIs, 65.7% reported immune-related AEs (irAEs); 96.1% reported mild-to-moderate irAEs. Cancer patients in China had a preliminary understanding of ICIs. Yet, the number of patients treated with ICIs was small.
Keywords: adverse effects; attitude; immunotherapy; practice; survey.
Copyright © 2021 Zhang, Wang, Zhang, Chu, Su, Wu, Chen, Wang, Yin, Zhu and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Chinese expert consensus recommendations for the administration of immune checkpoint inhibitors to special cancer patient populations.Ther Adv Med Oncol. 2023 Jul 16;15:17588359231187205. doi: 10.1177/17588359231187205. eCollection 2023. Ther Adv Med Oncol. 2023. PMID: 37484525 Free PMC article. Review.
-
Real-world safety experience with immune checkpoint inhibitors in Saudi Arabia.Sci Prog. 2021 Jan-Mar;104(1):36850421997302. doi: 10.1177/0036850421997302. Sci Prog. 2021. PMID: 33689534 Free PMC article.
-
Real-World Clinical and Economic Outcomes in Selected Immune-Related Adverse Events Among Patients with Cancer Receiving Immune Checkpoint Inhibitors.Oncologist. 2021 Nov;26(11):e2002-e2012. doi: 10.1002/onco.13918. Epub 2021 Aug 24. Oncologist. 2021. PMID: 34327774 Free PMC article.
-
Challenges and Limitations of Endocrine Toxicity Evaluation in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy-Retrospective Study from a Tertiary-Level Hospital in Romania.Diagnostics (Basel). 2023 May 18;13(10):1788. doi: 10.3390/diagnostics13101788. Diagnostics (Basel). 2023. PMID: 37238273 Free PMC article.
-
Ongoing Phase I Studies of Immune Checkpoint Inhibitors in China.Oncologist. 2019 Feb;24(Suppl 1):S11-S20. doi: 10.1634/theoncologist.2019-IO-S1-s03. Oncologist. 2019. PMID: 30819827 Free PMC article. Review.
Cited by
-
Regorafenib combined with programmed cell death-1 inhibitor against refractory colorectal cancer and the platelet-to-lymphocyte ratio's prediction on effectiveness.World J Gastrointest Oncol. 2022 Apr 15;14(4):920-934. doi: 10.4251/wjgo.v14.i4.920. World J Gastrointest Oncol. 2022. PMID: 35582108 Free PMC article.
-
Knowledge, attitudes and practices relating to nutritional support and immune-related adverse events among patients undergoing immunotherapy for liver cancer: cross-sectional study.BMJ Open. 2025 May 26;15(5):e086854. doi: 10.1136/bmjopen-2024-086854. BMJ Open. 2025. PMID: 40425243 Free PMC article.
References
-
- Dawe D. E., Christiansen D., Swaminath A., Ellis P. M., Rothney J., Rabbani R., et al. (2016). Chemoradiotherapy versus radiotherapy alone in elderly patients with stage III non-small cell lung cancer: a systematic review and meta-analysis. Lung Cancer 99, 180–185. 10.1016/j.lungcan.2016.07.016 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

