Proteomic Characterization, Biodistribution, and Functional Studies of Immune-Therapeutic Exosomes: Implications for Inflammatory Lung Diseases
- PMID: 33841418
- PMCID: PMC8027247
- DOI: 10.3389/fimmu.2021.636222
Proteomic Characterization, Biodistribution, and Functional Studies of Immune-Therapeutic Exosomes: Implications for Inflammatory Lung Diseases
Abstract
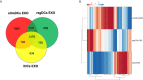
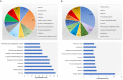
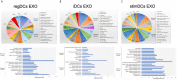
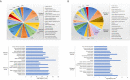
Dendritic cell (DC)-derived exosomes (DC EXO), natural nanoparticles of endosomal origin, are under intense scrutiny in clinical trials for various inflammatory diseases. DC EXO are eobiotic, meaning they are well-tolerated by the host; moreover, they can be custom-tailored for immune-regulatory or -stimulatory functions, thus presenting attractive opportunities for immune therapy. Previously we documented the efficacy of immunoregulatory DCs EXO (regDCs EXO) as immunotherapy for inflammatory bone disease, in an in-vivo model. We showed a key role for encapsulated TGFβ1 in promoting a bone sparing immune response. However, the on- and off-target effects of these therapeutic regDC EXO and how target signaling in acceptor cells is activated is unclear. In the present report, therapeutic regDC EXO were analyzed by high throughput proteomics, with non-therapeutic EXO from immature DCs and mature DCs as controls, to identify shared and distinct proteins and potential off-target proteins, as corroborated by immunoblot. The predominant expression in regDC EXO of immunoregulatory proteins as well as proteins involved in trafficking from the circulation to peripheral tissues, cell surface binding, and transmigration, prompted us to investigate how these DC EXO are biodistributed to major organs after intravenous injection. Live animal imaging showed preferential accumulation of regDCs EXO in the lungs, followed by spleen and liver tissue. In addition, TGFβ1 in regDCs EXO sustained downstream signaling in acceptor DCs. Blocking experiments suggested that sustaining TGFβ1 signaling require initial interaction of regDCs EXO with TGFβ1R followed by internalization of regDCs EXO with TGFβ1-TGFβ1R complex. Finally, these regDCs EXO that contain immunoregulatory cargo and showed biodistribution to lungs could downregulate the main severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) target receptor, ACE2 on recipient lung parenchymal cells via TGFβ1 in-vitro. In conclusion, these results in mice may have important immunotherapeutic implications for lung inflammatory disorders.
Keywords: COVID-19; dendritic cells; exosomes; immune therapy; lung diseases.
Copyright © 2021 Elashiry, Elsayed, Elashiry, Rashid, Ara, Arbab, Elawady, Hamrick, Liu, Zhi, Lucas, Vazquez and Cutler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Elashiry M, Elashiry MM, Elsayed R, Rajendran M, Auersvald C, Zeitoun R, et al. Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J Extracellular Vesicles (2020) 9:1795362. 10.1080/20013078.2020.1795362 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

