Melatonin Pretreatment Confers Heat Tolerance and Repression of Heat-Induced Senescence in Tomato Through the Modulation of ABA- and GA-Mediated Pathways
- PMID: 33841479
- PMCID: PMC8027311
- DOI: 10.3389/fpls.2021.650955
Melatonin Pretreatment Confers Heat Tolerance and Repression of Heat-Induced Senescence in Tomato Through the Modulation of ABA- and GA-Mediated Pathways
Abstract
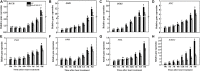
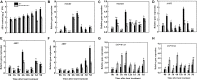
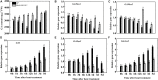
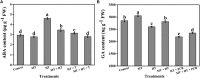
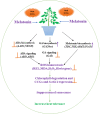
Heat stress and abscisic acid (ABA) induce leaf senescence, whereas melatonin (MT) and gibberellins (GA) play critical roles in inhibiting leaf senescence. Recent research findings confirm that plant tolerance to diverse stresses is closely associated with foliage lifespan. However, the molecular mechanism underlying the signaling interaction of MT with GA and ABA regarding heat-induced leaf senescence largely remains undetermined. Herein, we investigated putative functions of melatonin in suppressing heat-induced leaf senescence in tomato and how ABA and GA coordinate with each other in the presence of MT. Tomato seedlings were pretreated with 100 μM MT or water and exposed to high temperature (38/28°C) for 5 days (d). Heat stress significantly accelerated senescence, damage to the photosystem and upregulation of reactive oxygen species (ROS), generating RBOH gene expression. Melatonin treatment markedly attenuated heat-induced leaf senescence, as reflected by reduced leaf yellowing, an increased Fv/Fm ratio, and reduced ROS production. The Rbohs gene, chlorophyll catabolic genes, and senescence-associated gene expression levels were significantly suppressed by MT addition. Exogenous application of MT elevated the endogenous MT and GA contents but reduced the ABA content in high-temperature-exposed plants. However, the GA and ABA contents were inhibited by paclobutrazol (PCB, a GA biosynthesis inhibitor) and sodium tungstate (ST, an ABA biosynthesis inhibitor) treatment. MT-induced heat tolerance was compromised in both inhibitor-treated plants. The transcript abundance of ABA biosynthesis and signaling genes was repressed; however, the biosynthesis genes MT and GA were upregulated in MT-treated plants. Moreover, GA signaling suppressor and catabolic gene expression was inhibited, while ABA catabolic gene expression was upregulated by MT application. Taken together, MT-mediated suppression of heat-induced leaf senescence has collaborated with the activation of MT and GA biosynthesis and inhibition of ABA biosynthesis pathways in tomato.
Keywords: chlorophyll degradation; high temperature; leaf senescence; melatonin; tomato.
Copyright © 2021 Jahan, Shu, Wang, Hasan, El-Yazied, Alabdallah, Hajjar, Altaf, Sun and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ahammed G. J., Li Y., Cheng Y., Liu A., Chen S., Li X. (2020b). Abscisic acid and gibberellins act antagonistically to mediate epigallocatechin-3-gallate-retarded seed germination and early seedling growth in tomato. J. Plant Growth Regul. 39 1414–1424. 1–11. 10.1007/s00344-020-10089-1 - DOI
-
- Ahammed G. J., Xu W., Liu A., Chen S. (2019). Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 161 303–311. 10.1016/j.envexpbot.2018.06.006 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

