Safety and Pharmacokinetics of FMX103 (1.5% Minocycline Topical Foam) in Subjects with Moderate-to-Severe Papulopustular Rosacea under Maximum-use Treatment Conditions
- PMID: 33841618
- PMCID: PMC8021408
Safety and Pharmacokinetics of FMX103 (1.5% Minocycline Topical Foam) in Subjects with Moderate-to-Severe Papulopustular Rosacea under Maximum-use Treatment Conditions
Abstract
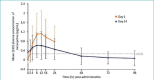
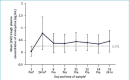
BACKGROUND: FMX103 1.5% is the first and only topical minocycline foam that is approved for the treatment of papulopustular rosacea in adults. OBJECTIVE: We sought to characterize the safety and pharmacokinetics of minocycline under maximal-use conditions of FMX103 1.5% in subjects with moderate-to-severe rosacea. METHODS: This Phase Isingle-center, nonrandomized, open-label, single-period pharmacokinetics and safety evaluation study evaluated multiple-dose, topical administration of FMX103 1.5%. Twenty subjects meeting study inclusion/exclusion criteria had ~2 grams of FMX103 1.5% applied to the full face once per day for 14 days. Blood samples were collected 30 minutes prior to study drug application on treatment Days 1, 2, 6, 9, 11, 12, and 14, and also at 2, 4, 8, 12, 16, and 24 hours post-administration on treatment Days 1 and 14. RESULTS: Following topical application of a 2-gram maximal-use dose of FMX103 1.5% for 14 days, minocycline plasma concentrations were low. Overall, trough levels were approximately 0.5ng/mL from 24 hours after the first dose through 24 hours after the last dose on Day 14, indicating that steady-state levels appear to have been reached within the first day of dosing. Daily application of FMX103 1.5% was generally safe and well-tolerated by all subjects. CONCLUSION: Once-daily topical application of FMX103 1.5% did not lead to appreciable systemic exposure or accumulation of minocycline, suggesting that it is a viable treatment option for papulopustular rosacea.
Keywords: Minocycline; Phase I study; maximum use; open-label; papulopustular rosacea; pharmacokinetics; topical foam.
Copyright © 2020. Matrix Medical Communications. All rights reserved.
Conflict of interest statement
FUNDING:This study was sponsored by Foamix Pharmaceuticals Ltd., a wholly owned subsidiary of VYNE Therapeutics Inc. DISCLOSURES:Dr. Jones served as the principal investigator on the study. Dr. Stuart is an employee and stockholder at VYNE Therapeutics Inc.
Figures

Similar articles
-
Efficacy and safety of topical minocycline preparations for papulopustular rosacea: a systematic review and meta-analysis.Front Med (Lausanne). 2025 Apr 1;12:1517825. doi: 10.3389/fmed.2025.1517825. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40236457 Free PMC article.
-
A Phase II, Randomized, Double-Blind Clinical Study Evaluating the Safety, Tolerability, and Efficacy of a Topical Minocycline Foam, FMX103, for the Treatment of Facial Papulopustular Rosacea.Am J Clin Dermatol. 2018 Jun;19(3):427-436. doi: 10.1007/s40257-017-0339-0. Am J Clin Dermatol. 2018. PMID: 29396702 Clinical Trial.
-
Open-label Extension Study Evaluating Long-term Safety and Efficacy of FMX103 1.5% Minocycline Topical Foam for the Treatment of Moderate-to-Severe Papulopustular Rosacea.J Clin Aesthet Dermatol. 2020 Nov;13(11):44-49. Epub 2020 Nov 1. J Clin Aesthet Dermatol. 2020. PMID: 33282103 Free PMC article.
-
Minocycline 1.5% foam for the topical treatment of moderate to severe papulopustular rosacea: Results of 2 phase 3, randomized, clinical trials.J Am Acad Dermatol. 2020 May;82(5):1166-1173. doi: 10.1016/j.jaad.2020.01.043. Epub 2020 Jan 28. J Am Acad Dermatol. 2020. PMID: 32004648 Clinical Trial.
-
Current topical and systemic approaches to treatment of rosacea.J Eur Acad Dermatol Venereol. 2009 Aug;23(8):876-82. doi: 10.1111/j.1468-3083.2009.03167.x. J Eur Acad Dermatol Venereol. 2009. PMID: 19508315 Review.
Cited by
-
Rosacea Topical Treatment and Care: From Traditional to New Drug Delivery Systems.Mol Pharm. 2023 Aug 7;20(8):3804-3828. doi: 10.1021/acs.molpharmaceut.3c00324. Epub 2023 Jul 21. Mol Pharm. 2023. PMID: 37478169 Free PMC article. Review.
-
Efficacy and safety of topical minocycline preparations for papulopustular rosacea: a systematic review and meta-analysis.Front Med (Lausanne). 2025 Apr 1;12:1517825. doi: 10.3389/fmed.2025.1517825. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40236457 Free PMC article.
-
Tetracyclines Revisited: Tetracyclines in the Field of Dermatology.Dermatology. 2024;240(5-6):844-858. doi: 10.1159/000542006. Epub 2024 Oct 18. Dermatology. 2024. PMID: 39427643 Free PMC article. Review.
References
-
- Oge LK, Muncie HL, Phillips-Savoy AR. Rosacea: diagnosis and treatment. Am Fam Physician. 2015;92(3):187–196. - PubMed
-
- Del Rosso JQ, Thiboutot D, Gallo R et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92(5):234–240. - PubMed
-
- Schaller M, Schofer H, Homey B et al. Rosacea Management: Update on general measures and topical treatment options. J Dtsch Dermatol Ges. 2016;14(6):17–27. - PubMed
LinkOut - more resources
Full Text Sources
