Mechanism of Cdk5-synaptophysin-SNARE pathway in acute and chronic inflammatory pain
- PMID: 33841641
- PMCID: PMC8014406
Mechanism of Cdk5-synaptophysin-SNARE pathway in acute and chronic inflammatory pain
Abstract
Purpose: Currently, there is no favorable treatment plan for inflammatory pain, so exploring new analgesics is still a research hotspot in this area. Cyclin-dependent protein kinase 5 (Cdk5) is a pain-related protein kinase, but its mechanism in inflammatory pain has not been clarified. This research aimed to explore the mechanism of Cdk5-synaptophysin (Syn)-soluble N-ethylmaleimide-sensitivity factor (NSF) attachment protein receptor (SNARE) in acute and chronic inflammatory pain.
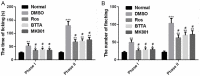
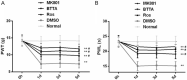
Methods: Rat models of acute and chronic inflammatory pain were induced by formalin and complete Freund's adjuvant (CFA), separately, and some rats injected with normal saline through intraplantar injection were divided into a control group. Thirty minutes before modeling, rats were given Cdk5 inhibitor (Roscovitine, Ros), SNARE scavenger (botulinum toxin A, BTTA), glutamate receptor inhibitor (MK801), and dimethyl sulfoxide (DMSO) through spinal canals, and the paw withdrawal threshold (PWT) and thermal withdrawal latency (PWL) at difference time points were compared.
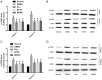
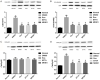
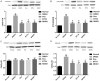
Results: Compared with rats in the control group, those in the rat models of acute and chronic inflammatory pain showed lower PWT and PWL, higher Cdk5 enzyme level, tight correlation of Cdk5 with Syn, SNARE, p25 proteins, and higher levels of Cdk5, Syn and SNARE. And the above situation was dramatically reversed under intervention of Ros, BTTA and MK801.
Conclusion: Cdk5-Syn-SNARE pathway is a therapeutic target for inflammatory pain. Blocking the activation of this pathway is beneficial to exert analgesic effect.
Keywords: Cdk5; Chronic inflammatory pain; SNARE; synaptophysin.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures
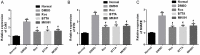

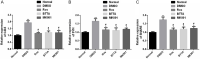
References
-
- Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol. 2018;39:240–255. - PubMed
-
- Ahmad N, Subhan F, Islam NU, Shahid M, Rahman FU, Fawad K. A novel pregabalin functionalized salicylaldehyde derivative afforded prospective pain, inflammation, and pyrexia alleviating propensities. Arch Pharm (Weinheim) 2017;350 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
