LASS2 mediates Nrf2-driven progestin resistance in endometrial cancer
- PMID: 33841656
- PMCID: PMC8014362
LASS2 mediates Nrf2-driven progestin resistance in endometrial cancer
Abstract
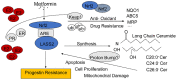
Progestin administration serves as the optimal conservative treatment method for women with endometrial cancer or precancer lesions who want to preserve fertility. However, there are still at least 30% of patients in which progestin resistance occurs. LASS2 (Ceramide Synthase 2) has been reported to be involved in chemotherapy resistance, whether it also plays a role in progestin resistance is not clear. Here, we explored the detailed mechanism by which Nrf2/LASS2 contributes to progestin resistance and disease progression.
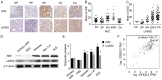
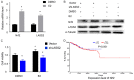
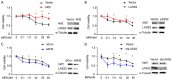
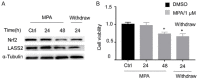
Methods: IHC assays were performed to estimate the expression pattern of Nrf2 and LASS2. Moreover, it bears three antioxidant response elements (ARE) in the promoter region of LASS2 gene, therefore, Luciferase assays were performed to determine if Nrf2 regulates LASS2 by binding with these ARE sequence. Western Blot assays were used to determine the expression of Nrf2 and LASS2 protein among various endometrial cell lines. Relative mRNA expression levels were detected by RT-PCR. Cellular growth was monitored with CCK-8 tests. Apoptosis was determined with Annexin V-PI staining and flow cytometry analysis. siRNA knockdown was performed to investigate the effects of Nrf2 on cell proliferation.
Result: Nrf2/LASS2 is highly expressed in endometrial cancer tissue, as compared to expression levels in normal endometrial tissue. Proliferation assays demonstrated that overexpression of Nrf2/LASS2 resulted in progestin resistance. Conversely, knockdown of LASS2 increased apoptosis and decreased cell viability. In addition, metformin overcame progestin resistance by down-regulating Nrf2/LASS2 expression.
Conclusion: Our findings provide new insight into the mechanism of progestin resistance in type I endometrial cancer. Nrf2/LASS2 may not only be a possible marker for predicting the prognosis of endometrial cancer but also serve as a potential therapeutic target.
Keywords: LASS2; Nrf2; Type I endometrial cancer; progestin resistance; proliferation.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures


References
-
- Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–1108. - PubMed
-
- Jerzak KJ, Duska L, Mackay HJ. Endocrine therapy in endometrial cancer: an old dog with new tricks. Gynecol Oncol. 2019;153:175–183. - PubMed
-
- Tomao F, Peccatori F, Del Pup L, Franchi D, Zanagnolo V, Panici PB, Colombo N. Special issues in fertility preservation for gynecologic malignancies. Crit Rev Oncol Hematol. 2016;97:206–219. - PubMed
-
- Pérez-Medina T, Bajo J, Folgueira G, Haya J, Ortega P. Atypical endometrial hyperplasia treatment with progestogens and gonadotropin-releasing hormone analogues: long-term follow-up. Gynecol Oncol. 1999;73:299–304. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
