PD-1/PDL-1 Inhibitors and Cardiotoxicity; Molecular, Etiological and Management Outlines
- PMID: 33842004
- PMCID: PMC8020146
- DOI: 10.1016/j.jare.2020.09.006
PD-1/PDL-1 Inhibitors and Cardiotoxicity; Molecular, Etiological and Management Outlines
Abstract
Background: The US Food and Drug Administration (FDA) has approved several immunotherapeutic drugs for cancer since 2010, and many more are still being evaluated in other clinical studies. These inhibitors significantly increase response rates and result in the treatment of patients with advanced cancer. However, cancer immunotherapy leads to essential cardiac toxicity properties that have become distinct from other cancer patients' care and are mostly related to their etiology.
Aim of review: As potential implications, the occurrence of cardiovascular adverse events is particularly challenging and needs a comprehensive understanding of overall cancer-related etiology, clinical outcomes with different variable severity, and management.
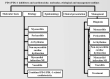
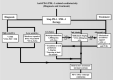
Key scientific concepts of review: In terms of improving the overall survival of patients with cancer, clinicians should be careful in selecting either programmed cell death-1 (PD-1) or its programmed cell death ligand (PDL-1) inhibitors by evaluating their risk and clinical benefit for early intervention and decrease the level of morbidity and mortality of their patients. This review focuses on the effectiveness of PD-1/PL-1 antibodies and associated cardiotoxicity adverse events, including etiological mechanisms, diagnosis, and treatment.
Keywords: Cardiotoxicity; Heart block; Myocarditis; PD-1; PDL-1.
© 2021 Cairo University.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
T cell checkpoint regulators in the heart.Cardiovasc Res. 2019 Apr 15;115(5):869-877. doi: 10.1093/cvr/cvz025. Cardiovasc Res. 2019. PMID: 30721928 Free PMC article. Review.
-
Immune Checkpoint Inhibitors and Cardiac Toxicity in Patients Treated for Non-Small Lung Cancer: A Review.Int J Mol Sci. 2020 Sep 29;21(19):7195. doi: 10.3390/ijms21197195. Int J Mol Sci. 2020. PMID: 33003425 Free PMC article. Review.
-
Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/ Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events.Eur J Cancer. 2020 Mar;128:17-26. doi: 10.1016/j.ejca.2019.12.031. Epub 2020 Mar 5. Eur J Cancer. 2020. PMID: 32109847
-
[Clinical predictive value of PD-1/PD-L1-induced electrocardiogram changes for cardiotoxicity].Zhonghua Zhong Liu Za Zhi. 2024 Oct 23;46(10):979-986. doi: 10.3760/cma.j.cn112152-20231024-00230. Zhonghua Zhong Liu Za Zhi. 2024. PMID: 39414599 Chinese.
-
Atypical autoimmune adverse effects with checkpoint blockade therapies.Ann Oncol. 2017 Feb 1;28(2):206-207. doi: 10.1093/annonc/mdw658. Ann Oncol. 2017. PMID: 27993802 No abstract available.
Cited by
-
Overall Survival in Heart Disease-Related Death in Non-Small Cell Lung Cancer Patients: Nonimmunotherapy Versus Immunotherapy Era: Population-Based Study.Front Oncol. 2020 Oct 22;10:572380. doi: 10.3389/fonc.2020.572380. eCollection 2020. Front Oncol. 2020. PMID: 33194672 Free PMC article.
-
Implication of the Gut Microbiome and Microbial-Derived Metabolites in Immune-Related Adverse Events: Emergence of Novel Biomarkers for Cancer Immunotherapy.Int J Mol Sci. 2023 Feb 1;24(3):2769. doi: 10.3390/ijms24032769. Int J Mol Sci. 2023. PMID: 36769093 Free PMC article. Review.
-
Salvage nivolumab and ipilimumab after prior anti-PD-1/PD-L1 therapy in metastatic renal cell carcinoma: A meta-analysis.Cancer Med. 2022 Apr;11(7):1669-1677. doi: 10.1002/cam4.4587. Epub 2022 Feb 9. Cancer Med. 2022. PMID: 35138046 Free PMC article.
-
Surgery improves survival in bladder signet-ring cell carcinoma-a population-based study.Ther Adv Urol. 2022 Apr 7;14:17562872221079473. doi: 10.1177/17562872221079473. eCollection 2022 Jan-Dec. Ther Adv Urol. 2022. PMID: 35422880 Free PMC article.
-
Immune checkpoint inhibitor (ICI) genes and aging in malignant melanoma patients: a clinicogenomic TCGA study.BMC Cancer. 2022 Sep 13;22(1):978. doi: 10.1186/s12885-022-09860-2. BMC Cancer. 2022. PMID: 36100891 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

