Endogenous sulfur dioxide is a novel inhibitor of hypoxia-induced mast cell degranulation
- PMID: 33842005
- PMCID: PMC8020161
- DOI: 10.1016/j.jare.2020.08.017
Endogenous sulfur dioxide is a novel inhibitor of hypoxia-induced mast cell degranulation
Abstract
Introduction: Mast cell (MC) degranulation is an important step in the pathogenesis of inflammatory reactions and allergies; however, the mechanism of stabilizing MC membranes to reduce their degranulation is unclear.
Methods: SO2 content in MC culture supernatant was measured by HPLC-FD. The protein and mRNA expressions of the key enzymes aspartate aminotransferase 1 (AAT1) and AAT2 and intracellular AAT activity were detected. The cAMP level in MCs was detected by immunofluorescence and ELISA. The release rate of MC degranulation marker β-hexosaminidase was measured. The expression of AAT1 and cAMP, the MC accumulation and degranulation in lung tissues were detected.
Objectives: To exam whether an endogenous sulfur dioxide (SO2) pathway exists in MCs and if it serves as a novel endogenous MC stabilizer.
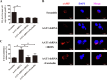
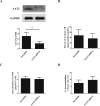
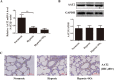
Results: We firstly show the existence of the endogenous SO2/AAT pathway in MCs. Moreover, when AAT1 was knocked down in MCs, MC degranulation was significantly increased, and could be rescued by a SO2 donor. Mechanistically, AAT1 knockdown decreased the cyclic adenosine monophosphate (cAMP) content in MCs, while SO2 prevented this reduction in a dose-independent manner. Pretreatment with the cAMP-synthesizing agonist forskolin or the cAMP degradation inhibitor IBMX significantly blocked the increase in AAT1 knockdown-induced MC degranulation. Furthermore, in hypoxia-stimulated MCs, AAT1 protein expression and SO2 production were markedly down regulated, and MC degranulation was activated, which were blunted by AAT1 overexpression. The cAMP synthesis inhibitor SQ22536 disrupted the suppressive effect of AAT1 overexpression on hypoxia-induced MC degranulation. In a hypoxic environment, mRNA and protein expression of AAT1 was significantly reduced in lung tissues of rats. Supplementation of SO2 elevated the cAMP level and reduced perivascular MC accumulation and degranulation in lung tissues of rats exposed to a hypoxic environment in vivo.
Conclusion: SO2 serves as an endogenous MC stabilizer via upregulating the cAMP pathway under hypoxic circumstance.
Keywords: Degranulation; Endogenous sulfur dioxide; Mast cells; Stabilization; cAMP.
© 2021 The Authors. Published by Elsevier B.V. on behalf of Cairo University.
Figures





Similar articles
-
Sulfur dioxide inhibits mast cell degranulation by sulphenylation of galectin-9 at cysteine 74.Front Immunol. 2024 Jun 17;15:1369326. doi: 10.3389/fimmu.2024.1369326. eCollection 2024. Front Immunol. 2024. PMID: 38953022 Free PMC article.
-
The Increased Endogenous Sulfur Dioxide Acts as a Compensatory Mechanism for the Downregulated Endogenous Hydrogen Sulfide Pathway in the Endothelial Cell Inflammation.Front Immunol. 2018 Apr 30;9:882. doi: 10.3389/fimmu.2018.00882. eCollection 2018. Front Immunol. 2018. PMID: 29760703 Free PMC article.
-
Endogenous sulfur dioxide is a novel adipocyte-derived inflammatory inhibitor.Sci Rep. 2016 Jun 1;6:27026. doi: 10.1038/srep27026. Sci Rep. 2016. PMID: 27246393 Free PMC article.
-
Potential therapeutic effect of SO₂ on fibrosis.Histol Histopathol. 2019 Dec;34(12):1289-1297. doi: 10.14670/HH-18-169. Epub 2019 Oct 11. Histol Histopathol. 2019. PMID: 31603240 Review.
-
Tetraspanins in the regulation of mast cell function.Med Microbiol Immunol. 2020 Aug;209(4):531-543. doi: 10.1007/s00430-020-00679-x. Epub 2020 Jun 7. Med Microbiol Immunol. 2020. PMID: 32507938 Free PMC article. Review.
Cited by
-
Effect of Natural Adenylcyclase/cAMP/CREB Signalling Activator Forskolin against Intra-Striatal 6-OHDA-Lesioned Parkinson's Rats: Preventing Mitochondrial, Motor and Histopathological Defects.Molecules. 2022 Nov 17;27(22):7951. doi: 10.3390/molecules27227951. Molecules. 2022. PMID: 36432051 Free PMC article.
-
Advances in the research of sulfur dioxide and pulmonary hypertension.Front Pharmacol. 2023 Oct 12;14:1282403. doi: 10.3389/fphar.2023.1282403. eCollection 2023. Front Pharmacol. 2023. PMID: 37900169 Free PMC article. Review.
-
A Whiff of Sulfur: One Wind a Day Keeps the Doctor Away.Antioxidants (Basel). 2022 May 24;11(6):1036. doi: 10.3390/antiox11061036. Antioxidants (Basel). 2022. PMID: 35739933 Free PMC article. Review.
-
Vascular smooth muscle cell-derived SO2 sulphenylated interferon regulatory factor 1 to inhibit VSMC senescence.Front Pharmacol. 2025 Mar 28;16:1516885. doi: 10.3389/fphar.2025.1516885. eCollection 2025. Front Pharmacol. 2025. PMID: 40223932 Free PMC article.
-
Recent advances on endogenous gasotransmitters in inflammatory dermatological disorders.J Adv Res. 2021 Sep 1;38:261-274. doi: 10.1016/j.jare.2021.08.012. eCollection 2022 May. J Adv Res. 2021. PMID: 35572410 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

