Hybrid Chemo-, Bio-, and Electrocatalysis for Atom-Efficient Deuteration of Cofactors in Heavy Water
- PMID: 33842020
- PMCID: PMC8025731
- DOI: 10.1021/acscatal.0c03437
Hybrid Chemo-, Bio-, and Electrocatalysis for Atom-Efficient Deuteration of Cofactors in Heavy Water
Abstract
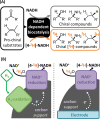
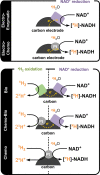
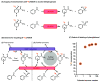
Deuterium-labeled nicotinamide cofactors such as [4-2H]-NADH can be used as mechanistic probes in biological redox processes and offer a route to the synthesis of selectively [2H] labeled chemicals via biocatalytic reductive deuteration. Atom-efficient routes to the formation and recycling of [4-2H]-NADH are therefore highly desirable but require careful design in order to alleviate the requirement for [2H]-labeled reducing agents. In this work, we explore a suite of electrode or hydrogen gas driven catalyst systems for the generation of [4-2H]-NADH and consider their use for driving reductive deuteration reactions. Catalysts are evaluated for their chemoselectivity, stereoselectivity, and isotopic selectivity, and it is shown that inclusion of an electronically coupled NAD+-reducing enzyme delivers considerable advantages over purely metal based systems, yielding exclusively [4S-2H]-NADH. We further demonstrate the applicability of these types of [4S-2H]-NADH recycling systems for driving reductive deuteration reactions, regardless of the facioselectivity of the coupled enzyme.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): A patent application detailing some of this research was filed through Oxford University Innovation (Feb 2018).
Figures


Similar articles
-
Bringing biocatalytic deuteration into the toolbox of asymmetric isotopic labelling techniques.Nat Commun. 2020 Mar 19;11(1):1454. doi: 10.1038/s41467-020-15310-z. Nat Commun. 2020. PMID: 32193396 Free PMC article.
-
Synthesis of [4S-2 H]NADH, [4R-2 H]NADH, [4-2 H2 ]NADH and [4-2 H]NAD+ cofactors through heterogeneous biocatalysis in heavy water.J Labelled Comp Radiopharm. 2021 Apr;64(4):181-186. doi: 10.1002/jlcr.3899. Epub 2021 Jan 26. J Labelled Comp Radiopharm. 2021. PMID: 33497029 Free PMC article.
-
Chemo-bio catalysis using carbon supports: application in H2-driven cofactor recycling.Chem Sci. 2021 May 7;12(23):8105-8114. doi: 10.1039/d1sc00295c. Chem Sci. 2021. PMID: 34194700 Free PMC article.
-
Recent Progress and Perspectives on Electrochemical Regeneration of Reduced Nicotinamide Adenine Dinucleotide (NADH).Chem Asian J. 2020 Dec 14;15(24):4256-4270. doi: 10.1002/asia.202001035. Epub 2020 Nov 19. Chem Asian J. 2020. PMID: 33164351 Review.
-
Catalyzing Electrosynthesis: A Homogeneous Electrocatalytic Approach to Reaction Discovery.Acc Chem Res. 2020 Mar 17;53(3):547-560. doi: 10.1021/acs.accounts.9b00529. Epub 2020 Feb 20. Acc Chem Res. 2020. PMID: 32077681 Free PMC article. Review.
Cited by
-
Catalytic asymmetric synthesis of enantioenriched α-deuterated pyrrolidine derivatives.Chem Sci. 2022 Mar 17;13(14):4041-4049. doi: 10.1039/d2sc00826b. eCollection 2022 Apr 6. Chem Sci. 2022. PMID: 35440992 Free PMC article.
-
Controlled Biocatalytic Synthesis of a Metal Nanoparticle-Enzyme Hybrid: Demonstration for Catalytic H2-driven NADH Recycling.Angew Chem Int Ed Engl. 2024 Jul 1;63(27):e202404024. doi: 10.1002/anie.202404024. Epub 2024 May 28. Angew Chem Int Ed Engl. 2024. PMID: 38641561 Free PMC article.
-
Light-driven decarboxylative deuteration enabled by a divergently engineered photodecarboxylase.Nat Commun. 2021 Jun 25;12(1):3983. doi: 10.1038/s41467-021-24259-6. Nat Commun. 2021. PMID: 34172745 Free PMC article.
-
Role of geochemical protoenzymes (geozymes) in primordial metabolism: specific abiotic hydride transfer by metals to the biological redox cofactor NAD.FEBS J. 2022 Jun;289(11):3148-3162. doi: 10.1111/febs.16329. Epub 2022 Jan 3. FEBS J. 2022. PMID: 34923745 Free PMC article.
-
Synergy of metal nanoparticles and organometallic complex in NAD(P)H regeneration via relay hydrogenation.Nat Commun. 2022 Sep 28;13(1):5699. doi: 10.1038/s41467-022-33312-x. Nat Commun. 2022. PMID: 36171210 Free PMC article.
References
-
- Singh P.; Guo Q.; Kohen A. Chemoenzymatic Synthesis of Ubiquitous Biological Redox Cofactors. Synlett 2017, 28 (10), 1151–1159. 10.1055/s-0036-1588768. - DOI
-
- Wong C. H.; Whitesides G. M. Enzyme-Catalyzed Organic Synthesis: Regeneration of Deuterated Nicotinamide Cofactors for Use in Large-Scale Enzymatic Synthesis of Deuterated Substances. J. Am. Chem. Soc. 1983, 105 (15), 5012–5014. 10.1021/ja00353a026. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
