Comprehensive Comparison Between Adjuvant Targeted Therapy and Chemotherapy for EGFR-Mutant NSCLC Patients: A Cost-Effectiveness Analysis
- PMID: 33842322
- PMCID: PMC8027108
- DOI: 10.3389/fonc.2021.619376
Comprehensive Comparison Between Adjuvant Targeted Therapy and Chemotherapy for EGFR-Mutant NSCLC Patients: A Cost-Effectiveness Analysis
Abstract
Background: Chemotherapy has been the current standard adjuvant treatment for early-stage non-small-cell lung cancer (NSCLC) patients, while recent studies showed benefits of epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI). We conducted a cost-effectiveness analysis to comprehensively evaluate the benefit of EGFR-TKI compared with chemotherapy for early-stage EGFR-mutant NSCLC patients after resection from the perspective of the Chinese health care system.
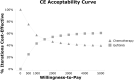
Method: A Markov model was established. Clinical data were based on the phase 3, ADJUVANT trial, where stage II-IIIA, EGFR-mutant NSCLC patients were randomized into gefitinib group or chemotherapy group after resection. Cost parameters mainly included costs of drugs, examinations, and adverse events (AEs). Effect parameters were evaluated by quality-adjusted life year (QALY). Outcomes contained incremental cost-effective ratio (ICER), average cost-effective ratio (ACER), and net benefit. The willingness-to-pay threshold was set as 3 times per capita gross domestic product ($30,828/QALY). Sensitivity analyses were also conducted to verify the stability of the model.
Results: Patients who received gefitinib had both a higher cost ($12,057.98 vs. $11,883.73) and a higher QALY (1.55 vs. 1.42) than patients who received chemotherapy. With an ICER of $1,345.62/QALY, adjuvant gefitinib was of economic benefit compared with chemotherapy. The ACER and net benefit were also consistent (gefitinib vs. chemotherapy, ACER: $7,802.30/QALY vs. $8,392.77/QALY; net benefit: $35,584.85 vs. $31,767.17). Sensitivity analyses indicated the stability of the model and the impact of utility.
Conclusion: Adjuvant EGFR-TKI application for early-stage EGFR-mutant NSCLC patients was cost-effective compared with chemotherapy, which might provide a reference for clinical decision-making and medical insurance policy formulation in China.
Keywords: adjuvant therapy; chemotherapy; cost-effectiveness analysis; epidermal growth factor receptor-tyrosine kinase inhibitor; non-small-cell lung cancer.
Copyright © 2021 Li, Guo, Li and Cui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous