Epigallocathechin- O-3-Gallate Inhibits Trypanothione Reductase of Leishmania infantum, Causing Alterations in Redox Balance and Leading to Parasite Death
- PMID: 33842389
- PMCID: PMC8027256
- DOI: 10.3389/fcimb.2021.640561
Epigallocathechin- O-3-Gallate Inhibits Trypanothione Reductase of Leishmania infantum, Causing Alterations in Redox Balance and Leading to Parasite Death
Abstract
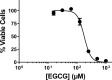
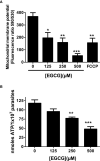
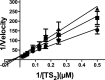
Leishmania infantum is a protozoan parasite that causes a vector borne infectious disease in humans known as visceral leishmaniasis (VL). This pathology, also caused by L. donovani, presently impacts the health of 500,000 people worldwide, and is treated with outdated anti-parasitic drugs that suffer from poor treatment regimens, severe side effects, high cost and/or emergence of resistant parasites. In previous works we have disclosed the anti-Leishmania activity of (-)-Epigallocatechin 3-O-gallate (EGCG), a flavonoid compound present in green tea leaves. To date, the mechanism of action of EGCG against Leishmania remains unknown. This work aims to shed new light into the leishmanicidal mode of action of EGCG. Towards this goal, we first confirmed that EGCG inhibits L. infantum promastigote proliferation in a concentration-dependent manner. Second, we established that the leishmanicidal effect of EGCG was associated with i) mitochondria depolarization and ii) decreased concentration of intracellular ATP, and iii) increased concentration of intracellular H2O2. Third, we found that the leishmanicidal effect and the elevated H2O2 levels induced by of EGCG can be abolished by PEG-catalase, strongly suggesting that this flavonoid kills L. infantum promastigotes by disturbing their intracellular redox balance. Finally, we gathered in silico and in vitro evidence that EGCG binds to trypanothione reductase (TR), a central enzyme of the redox homeostasis of Leishmania, acting as a competitive inhibitor of its trypanothione substrate.
Keywords: EGCG; Leishmania infantum; competitive inhibitor; mechanism of action; trypanothione reductase.
Copyright © 2021 Inacio, Fonseca, Limaverde-Sousa, Tomas, Castro and Almeida-Amaral.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Baiocco P., Poce G., Alfonso S., Cocozza M., Porretta G. C., Colotti G., et al. (2013). Inhibition of Leishmania infantum trypanothione reductase by azole-based compounds: A comparative analysis with its physiological substrate by x-ray crystallography. ChemMedChem 8, 1175–1183. 10.1002/cmdc.201300176 - DOI - PubMed
-
- Barral A., Pedral-Sampaio D., Grimaldi Junior G., Momen H., McMahon-Pratt D., Ribeiro de Jesus A., et al. (1991). Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am. J. Trop. Med. Hyg. 44 (5), 536–546. 10.4269/ajtmh.1991.44.536 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

