The Anti-Inflammatory Effect and Mucosal Barrier Protection of Clostridium butyricum RH2 in Ceftriaxone-Induced Intestinal Dysbacteriosis
- PMID: 33842393
- PMCID: PMC8027357
- DOI: 10.3389/fcimb.2021.647048
The Anti-Inflammatory Effect and Mucosal Barrier Protection of Clostridium butyricum RH2 in Ceftriaxone-Induced Intestinal Dysbacteriosis
Abstract
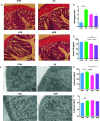
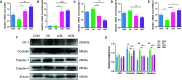
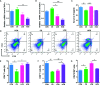
This study aimed at determining the beneficial effect of Clostridium butyricum (CB) RH2 on ceftriaxone-induced dysbacteriosis. To this purpose, BALB/c mice were exposed to ceftriaxone (400 mg/ml) or not (control) for 7 days, and administered a daily oral gavage of low-, and high-dose CB RH2 (108 and 1010 CFU/ml, respectively) for 2 weeks. CB RH2 altered the diversity of gut microbiota, changed the composition of gut microbiota in phylum and genus level, decreased the F/B ratio, and decreased the pro-inflammatory bacteria (Deferribacteres, Oscillibacter, Desulfovibrio, Mucispirillum and Parabacteroides) in ceftriaxone-treated mice. Additionally, CB RH2 improved colonic architecture and intestinal integrity by improving the mucous layer and the tight junction barrier. Furthermore, CB RH2 also mitigated intestinal inflammation through decreasing proinflammatory factors (TNF-α and COX-2) and increasing anti-inflammatory factors (IL-10). CB RH2 had direct effects on the expansion of CD4+ T cells in Peyer's patches (PPs) in vitro, which in turn affected their immune response upon challenge with ceftriaxone. All these data suggested that CB RH2 possessed the ability to modulate the intestinal mucosal and systemic immune system in limiting intestinal alterations to relieve ceftriaxone-induced dysbacteriosis.
Keywords: CB RH2 in Intestinal Dysbiosis; Clostridium butyricum; gut microbiota; immune response; mucosal barrier function.
Copyright © 2021 Li, Liu, Liu, Sui, Liu, Wei, Liu, Cheng, Ye, Gao, Wang, Lu, Cheng, Zhang, Yuan and Li.
Conflict of interest statement
Authors HC and LZ were employed by the company Hangzhou Grand Biologic Pharmaceutical INC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Probiotic Clostridium butyricum Improves the Growth Performance, Immune Function, and Gut Microbiota of Weaning Rex Rabbits.Probiotics Antimicrob Proteins. 2019 Dec;11(4):1278-1292. doi: 10.1007/s12602-018-9476-x. Probiotics Antimicrob Proteins. 2019. PMID: 30324399
-
Effects of ceftriaxone induced intestinal dysbacteriosis on lymphocytes in different tissues in mice.Immunobiology. 2016 Sep;221(9):994-1000. doi: 10.1016/j.imbio.2016.04.003. Epub 2016 May 7. Immunobiology. 2016. PMID: 27256989
-
Clostridium Butyricum ZJU-F1 Benefits the Intestinal Barrier Function and Immune Response Associated with Its Modulation of Gut Microbiota in Weaned Piglets.Cells. 2021 Mar 2;10(3):527. doi: 10.3390/cells10030527. Cells. 2021. PMID: 33801396 Free PMC article.
-
Clostridium butyricum, a future star in sepsis treatment.Front Cell Infect Microbiol. 2024 Dec 6;14:1484371. doi: 10.3389/fcimb.2024.1484371. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39711782 Free PMC article. Review.
-
[Physiological patterns of intestinal microbiota. The role of dysbacteriosis in obesity, insulin resistance, diabetes and metabolic syndrome].Orv Hetil. 2016 Jan 3;157(1):13-22. doi: 10.1556/650.2015.30296. Orv Hetil. 2016. PMID: 26708682 Review. Hungarian.
Cited by
-
Short-term exposure to antibiotics begets long-term disturbance in gut microbial metabolism and molecular ecological networks.Microbiome. 2024 May 7;12(1):80. doi: 10.1186/s40168-024-01795-z. Microbiome. 2024. PMID: 38715137 Free PMC article.
-
Fecal Microbiota Transplantation from Mice Receiving Magnetic Mitohormesis Treatment Reverses High-Fat Diet-Induced Metabolic and Osteogenic Dysfunction.Int J Mol Sci. 2025 Jun 6;26(12):5450. doi: 10.3390/ijms26125450. Int J Mol Sci. 2025. PMID: 40564914 Free PMC article.
-
Clostridium butyricum RH2 Alleviates Chronic Foot Shock Stress-Induced Behavioral Deficits in Rats via PAI-1.Front Pharmacol. 2022 Apr 6;13:845221. doi: 10.3389/fphar.2022.845221. eCollection 2022. Front Pharmacol. 2022. PMID: 35462923 Free PMC article.
-
Fecal microbiota transplantation attenuates Alzheimer's disease symptoms in APP/PS1 transgenic mice via inhibition of the TLR4-MyD88-NF-κB signaling pathway-mediated inflammation.Behav Brain Funct. 2025 Jan 8;21(1):2. doi: 10.1186/s12993-024-00265-8. Behav Brain Funct. 2025. PMID: 39780269 Free PMC article.
-
Octenyl Succinic Anhydride-Modified Starch Attenuates Body Weight Gain and Changes Intestinal Environment of High-Fat Diet-Fed Mice.Foods. 2022 Sep 23;11(19):2980. doi: 10.3390/foods11192980. Foods. 2022. PMID: 36230056 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

