From Flies to Men: ROS and the NADPH Oxidase in Phagocytes
- PMID: 33842458
- PMCID: PMC8033005
- DOI: 10.3389/fcell.2021.628991
From Flies to Men: ROS and the NADPH Oxidase in Phagocytes
Abstract
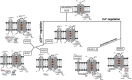
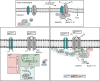
The cellular formation of reactive oxygen species (ROS) represents an evolutionary ancient antimicrobial defense system against microorganisms. The NADPH oxidases (NOX), which are predominantly localized to endosomes, and the electron transport chain in mitochondria are the major sources of ROS. Like any powerful immunological process, ROS formation has costs, in particular collateral tissue damage of the host. Moreover, microorganisms have developed defense mechanisms against ROS, an example for an arms race between species. Thus, although NOX orthologs have been identified in organisms as diverse as plants, fruit flies, rodents, and humans, ROS functions have developed and diversified to affect a multitude of cellular properties, i.e., far beyond direct antimicrobial activity. Here, we focus on the development of NOX in phagocytic cells, where the so-called respiratory burst in phagolysosomes contributes to the elimination of ingested microorganisms. Yet, NOX participates in cellular signaling in a cell-intrinsic and -extrinsic manner, e.g., via the release of ROS into the extracellular space. Accordingly, in humans, the inherited deficiency of NOX components is characterized by infections with bacteria and fungi and a seemingly independently dysregulated inflammatory response. Since ROS have both antimicrobial and immunomodulatory properties, their tight regulation in space and time is required for an efficient and well-balanced immune response, which allows for the reestablishment of tissue homeostasis. In addition, distinct NOX homologs expressed by non-phagocytic cells and mitochondrial ROS are interlinked with phagocytic NOX functions and thus affect the overall redox state of the tissue and the cellular activity in a complex fashion. Overall, the systematic and comparative analysis of cellular ROS functions in organisms of lower complexity provides clues for understanding the contribution of ROS and ROS deficiency to human health and disease.
Keywords: CGD; NADPH; inflammation; macrophages; mitochondrial ROS; myeloid cells; neutrophils; reactive oxygen species.
Copyright © 2021 Moghadam, Henneke and Kolter.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

