3-Deoxysappanchalcone Inhibits Skin Cancer Proliferation by Regulating T-Lymphokine-Activated Killer Cell-Originated Protein Kinase in vitro and in vivo
- PMID: 33842463
- PMCID: PMC8027363
- DOI: 10.3389/fcell.2021.638174
3-Deoxysappanchalcone Inhibits Skin Cancer Proliferation by Regulating T-Lymphokine-Activated Killer Cell-Originated Protein Kinase in vitro and in vivo
Abstract
Background: Skin cancer is one of the most commonly diagnosed cancers worldwide. The 5-year survival rate of the most aggressive late-stage skin cancer ranges between 20 and 30%. Thus, the discovery and investigation of novel target therapeutic agents that can effectively treat skin cancer is of the utmost importance. The T-lymphokine-activated killer cell-originated protein kinase (TOPK), which belongs to the serine-threonine kinase class of the mitogen-activated protein kinase kinase (MAPKK) family, is highly expressed and activated in skin cancer. The present study investigates the role of 3-deoxysappanchalcone (3-DSC), a plant-derived functional TOPK inhibitor, in suppressing skin cancer cell growth.
Purpose: In the context of skin cancer prevention and therapy, we clarify the effect and mechanism of 3-DSC on different types of skin cancer and solar-simulated light (SSL)-induced skin hyperplasia.
Methods: In an in vitro study, western blotting and in vitro kinase assays were utilized to determine the protein expression of TOPK and its activity, respectively. Pull-down assay with 3-DSC and TOPK (wild-type and T42A/N172 mutation) was performed to confirm the direct interaction between T42A/N172 amino acid sites of TOPK and 3-DSC. Cell proliferation and anchorage-independent cell growth assays were utilized to determine the effect of 3-DSC on cell growth. In an in vivo study, the thickness of skin and tumor size were measured in the acute SSL-induced inflammation mouse model or SK-MEL-2 cell-derived xenografts mouse model treated with 3-DSC. Immunohistochemistry analysis of tumors isolated from SK-MEL-2 cell-derived xenografts was performed to determine whether cell-based results observed upon 3-DSC treatment could be recapitulated in vivo.
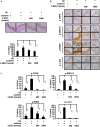
Results: 3-DSC is able to inhibit cell proliferation in skin cancer cells in an anchorage-dependent and anchorage-independent manner by regulation of TOPK and its related signaling pathway in vitro. We also found that application of 3-DSC reduced acute SSL-induced murine skin hyperplasia. Additionally, we observed that 3-DSC decreased SK-MEL-2 cell-derived xenograft tumor growth through attenuating phosphorylation of TOPK and its downstream effectors including ERK, RSK, and c-Jun.
Conclusions: Our results suggest that 3-DSC may function in a chemopreventive and chemotherapeutic capacity by protecting against UV-induced skin hyperplasia and inhibiting tumor cell growth by attenuating TOPK signaling, respectively.
Keywords: 3-deoxysappanchalcone; T-LAK cell-originated protein kinase; cancer growth; skin cancer; skin hyperplasia; solar sinulated light.
Copyright © 2021 Fu, Zhao, Yoon, Shim, Choi, Yin, Xu, Laster, Liu, Dong and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Abe Y., Matsumoto S., Kito K., Ueda N. (2000). Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J. Biol. Chem. 275 21525–21531. 10.1074/jbc.m909629199 - DOI - PubMed
-
- Bermudez Y., Stratton S. P., Curiel-Lewandrowski C., Warneke J., Hu C., Bowden G. T., et al. (2015). Activation of the PI3K/Akt/mTOR and MAPK signaling pathways in response to acute solar-simulated light exposure of human skin. Cancer Prev. Res. (Phila) 8 720–728. 10.1158/1940-6207.capr-14-0407 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

