Mechanisms of Oocyte Maturation and Related Epigenetic Regulation
- PMID: 33842483
- PMCID: PMC8025927
- DOI: 10.3389/fcell.2021.654028
Mechanisms of Oocyte Maturation and Related Epigenetic Regulation
Abstract
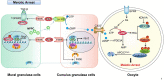
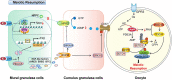
Meiosis is the basis of sexual reproduction. In female mammals, meiosis of oocytes starts before birth and sustains at the dictyate stage of meiotic prophase I before gonadotropins-induced ovulation happens. Once meiosis gets started, the oocytes undergo the leptotene, zygotene, and pachytene stages, and then arrest at the dictyate stage. During each estrus cycle in mammals, or menstrual cycle in humans, a small portion of oocytes within preovulatory follicles may resume meiosis. It is crucial for females to supply high quality mature oocytes for sustaining fertility, which is generally achieved by fine-tuning oocyte meiotic arrest and resumption progression. Anything that disturbs the process may result in failure of oogenesis and seriously affect both the fertility and the health of females. Therefore, uncovering the regulatory network of oocyte meiosis progression illuminates not only how the foundations of mammalian reproduction are laid, but how mis-regulation of these steps result in infertility. In order to provide an overview of the recently uncovered cellular and molecular mechanism during oocyte maturation, especially epigenetic modification, the progress of the regulatory network of oocyte meiosis progression including meiosis arrest and meiosis resumption induced by gonadotropins is summarized. Then, advances in the epigenetic aspects, such as histone acetylation, phosphorylation, methylation, glycosylation, ubiquitination, and SUMOylation related to the quality of oocyte maturation are reviewed.
Keywords: meiosis arrest; meiosis resumption; oocyte; oocyte maturation; ovary.
Copyright © 2021 He, Zhang, Yang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

